Serum branch chain amino acids and aromatic amino acids ratio and metabolic risks in Koreans with normal-weight or obesity: a cross-sectional study
Article information
Abstract
Objectives
Metabolic disease is strongly associated with future insulin resistance, and its prevalence is increasing worldwide. Thus, identifying early biomarkers of metabolic-related disease based on serum profiling is useful to control future metabolic disease. Our study aimed to assess the association of serum branched chain amino acids (BCAAs) and aromatic amino acids (AAAs) ratio and metabolic disease according to body mass index (BMI) status among Korean adults.
Methods
This cross-sectional study included 78 adults aged 20–59 years in Korea. We compared serum amino acid (AA) levels between adults with normal-weight and adults with obesity and investigated biomarkers of metabolic disease. We examined serum AA levels, blood profile, and body composition. We also evaluated the association between serum AAs and metabolic-related disease.
Results
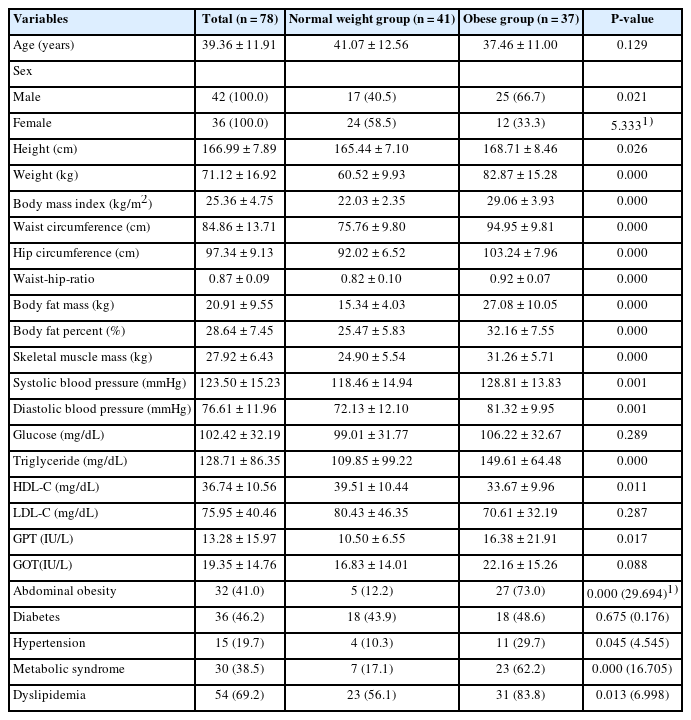
The height, weight, BMI, waist circumference, hip circumference, waist-hip-ratio, body fat mass, body fat percent, skeletal muscle mass, systolic blood pressure, and diastolic blood pressure were higher in the group with obesity compared to normal weight group. The group with obesity showed significantly higher levels of BCAA, AAA, and BCAA and AAA ratio. Further, BCAA and AAA ratio were significantly positively correlated with triglyceride, body weight, and skeletal muscle mass. The evaluation of metabolic disease risks revealed an association between the ratios of BCAAs and AAAs, hypertension, and metabolic syndrome.
Conclusions
Our study is showed the associations between BCAA and AAA ratio, obesity, and obesity-related diseases using various analytical approaches. The elevated BCAA and AAA ratio could be early biomarkers for predicting future metabolic diseases in Korean population.
INTRODUCTION
Metabolic diseases known as pathophysiological disorders that include diabetes mellitus (DM), hypertension, dyslipidemia, and metabolic syndrome often result in cardiovascular disease or stroke [1]. The rate of metabolic disease has increased dramatically in the Korean population due to a shift towards a Western diet and sedentary lifestyle [2]. The development of valuable biomarkers is essential for the early detection of metabolic diseases in order to prevent the diseases’ progression and exacerbation patients’ conditions [3].
Amino acids (AAs) are the structural units of proteins with important roles in gene expression, cell signaling, reproduction, metabolism, and oxidative stress [4]. The human body can synthesize several AA, including non-essential AA such as alanine, aspartate, and glutamate [5]. The precursors of dopamine, epinephrine, norepinephrine, serotonin, and thyroxine are the aromatic amino acid (AAA), which include histidine, phenylalanine, tryptophan, and tyrosine. Their side chains feature an aromatic ring [4]. The serum branched chain amino acids (BCAAs) include leucine, isoleucine, and valine; these share a structurally similar side chain. The AAA and BCAA known as essential AA, account for 40% of the total amount AA required by mammals including humans. These AA are crucial for proper metabolism and must be obtained through diet [6]. BCAA are important for protein/muscle preservation in the human body [7] and its supplementation has beneficial effects on protein turnover and muscle wasting in patients with liver cirrhosis, renal failure, liver cancer, and sepsis [8,9]. According to a recent report, the serum AA profile can serve as an effective biomarker for the detection of metabolic disease [10]. The serum concentrations of BCAA and AAA were shown to be related to the risk of future hyperglycemia and diabetes [11,12].
Previous studies reported the potential of serum free AAs for the diagnosis of type 2 diabetes mellitus (T2DM) [13]. One study has highlighted that the serum levels of BCAAs and AAAs are significantly correlated with insulin resistance in T2DM and may improve insulin secretion or modulate insulin sensitivity [14]. Increased levels of AAA and BCAA reduce the skeletal muscles’ ability to oxidize glucose, which leads to insulin resistance and the depletion of pancreatic islet cells. Particularly in patients who are pre-diabetic, BCAA and AAAs may be regarded as indicators [15].
A prospective cohort study showed significant relationship of increased serum BCAA and AAA with a higher risk of developing insulin resistance [16]. According to a large cross-sectional research, obesity affected the direct relationships between BCAA and AAA and insulin resistance, with the connections being more significant in women [11]. More recently, a study showed variations in the blood levels of these AAs among different ethnic groups, and it suggested that these variations could help understand why South Asian populations have a higher risk of diabetes than European populations [17]. According to several recently published research, there is a strong and independent relationship between hypertension and altered levels of AAA and BCAA [18]. However, the relationship between serum AAs and obesity in the development of metabolic disease has not been investigated in Korean ethnicities who are generally less obese and more insulin-resistant at the same status of body mass index (BMI) than Caucasians.
Therefore, this study aimed to assess the association of BCAA and AAA ratio and various metabolic disease according to BMI status among Korean adults. We hypothesize that there is a significant relationship of serum BCAA and AAA and these ratio with the BMI status, and that the relationship of AA with metabolic risks is more pronounced among individuals with obesity than among normal-weight individuals.
METHODS
Ethics statement
The research was carried out in compliance with the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Boards of Changwon National University (approval No. 104027-201706-HR-008) and Changwon Fatima Hospital (approval No. 17-04). Informed consent was obtained from all participants.
1. Participants
This study was conducted from April 2017 to July 2018 at Changwon, South Korea. The cross-sectional study included 78 participants who were recruited from Changwon National University and Changwon Fatima Hospital. The 78 participants were self-enrolled in this study; their age ranged between 20 and 59 years. Posters, blogs, and social media networks were among the offline and online mediums use to accomplish recruitment. Potential participants performed phone screening before eligibility was established during a screening visit. We excluded participants with a history of cardiac disease, renal disease, hepatic infections; those with thyroid function, or malignancies, consumptive disease; or pregnant and lactating patients. We included adults aged 20 to 59 years without any disease that met the exclusion criteria.
2. Anthropometric measurement
Anthropometric measurements were obtained using bioelectrical impedance analysis (InBody 720 Body Composition Analyzer; Bio Space Co., Seoul, Korea). The participants were measured in the biped position, after a rest for approximately 3 minutes. The participants were wearing loose clothes without footwear prior to measurement. Height and weight were measured, and BMI was calculated. The participants’ arms were spread wide while they stood for the measurements of their waist and hip circumferences. All circumferences were obtained with a measuring tape. Anthropometric measurements were taken twice, and the average results were recorded with a precision of 2 decimal places.
3. Measurement of metabolic variables and serum AAs
Blood samples were collected from the participants after an 8-hour fast. Blood samples were centrifuged for 15 minutes at 1,500 rpm to separate serum. Serum samples were stored at −80°C until analysis. Serum levels of triglyceride (TG), total cholesterol (To-C), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured enzymatically. Fasting glucose was measured with the hexokinase method, and insulin was determined using the chemiluminescence immunoassay. Serum glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT) levels were measured following the method of Reitman and Frankel using assay kit (Asan Corp., Seoul, Korea). Serum AAs were measured by high-performance liquid chromatography-mass spectrometry. Analysis was performed by using the AA analyzer (SYKAM Corp., Seoul, Korea). We targeted 3 BCAA (isoleucine, leucine, and valine) and 2 AAAs (phenylalanine, tyrosine), referred to the BCAA and AAA ratio literature [19,20]. Concentrations of AA were expressed in mg/100 g.
4. Clinical assessments
In this study, metabolic syndrome was determined according to the following Korean diagnostic criteria: at least 3 of the following 5 components: (1) abdominal obesity (waist ≥ 90 cm in males and ≥ 80 cm in females); (2) HDL-C < 40 mg/dL in males and HDL-C < 50 mg/dL in females; (3) TG ≥ 150 mg/dL, or the use of medication for dyslipidemia; (4) fasting glucose ≥ 100 mg/dL or the use of medication for DM; and (5) blood pressure ≥ 130/85 mmHg or the use of antihypertensive medication [16].
T2DM was diagnosed in patients with a fasting glucose level of 126 mg/dL or higher, a hemoglobin A1c level of 6.5% or higher or taking medication for DM [17]. Dyslipidemia was diagnosed in individuals with a To-C level of 240 mg/dL or higher, an LDL-C level of 160 mg/dL or higher, an HDL-C level of less than 40 mg/dL, a TG level of 200 mg/dL or higher or taking medication for dyslipidemia [18]. Hypertension was diagnosed in patients with a systolic blood pressure (SBP) of 140 mmHg or higher, a diastolic blood pressure (DBP) of 90 mmHg or higher or taking medication for hypertension [19].
5. Statistical analysis
The results are expressed as mean ± standard deviation for continuous data. IBM SPSS Statistics for Windows, version 25 (IBM Corporation, Armonk, NY, USA) was used for the analysis of the data. We compared the characteristics between anthropometrics and blood samples using χ2 tests for categorical variables and t-tests for continuous variables. Multiple linear regression analysis was used to evaluate correlations of serum AAs with each metabolic variable. Correlation covariance value (β) and adjusted coefficient determination (r2) were estimated. To evaluate the association between serum AA and metabolic disease risk, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) for metabolic disease in relation to serum AA using conditional logistic regression. Tertile cutoff points for were analyzed based on the distribution of serum AA in comparison groups. Value is calculated that ORs and 95% CIs as a categorical variable for developing metabolic syndrome, dyslipidemia, or hypertension from logistic regressions. Statistical differences were considered significant based on a P-value of < 0.05.
RESULTS
1. Demographic and clinical characteristics
The numbers of participants and their baseline characteristics are presented in Table 1. No statistically significant differences in age and levels of glucose, LDL-C, and GOT were noted between the normal weight group and group with obesity. As expected, height, weight, BMI, waist circumference, hip circumference, waist-hip-ratio, body fat mass, body fat percent, skeletal muscle mass, SBP, and DBP were higher in the group with obesity compared to normal weight group (P < 0.01). Participants with obesity had higher blood concentration of TG, and GPT compared to the normal weight group (P < 0.05). HDL-C was higher in normal weight group than obesity group (P < 0.05). The prevalence of abdominal obesity, hypertension, and dyslipidemia were higher in the group with obesity than in the normal weight group (P < 0.05). The prevalence of T2DM was not significantly different between the 2 groups.
2. Serum BCAA and AAA
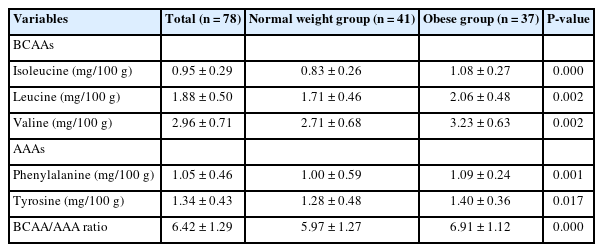
Serum AA concentrations for the study participants are presented in Table 2. There was a significant difference in the total concentration of serum AA in the total group. All serum BCAA and AAA levels were elevated in the group with obesity, while it was significantly decreased in the normal weight group (P < 0.01). A significant difference in the serum BCAA and AAA ratio was found between the 2 groups (P < 0.001); the serum BCAA and AAA ratio was significantly higher in the group with obesity.
3. Correlation between BCAA and AAA ratio and metabolic variables
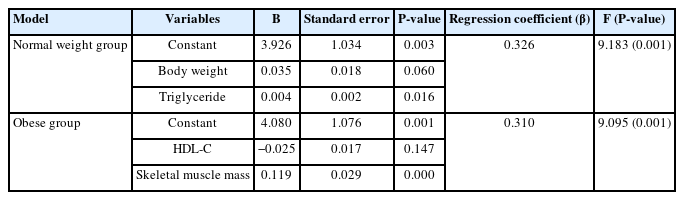
We examined the association between metabolic variables and BCAA and AAA ratio measured in this study (Table 3). We conducted multiple linear regression analysis to identify the relationship between BCAA and AAA ratio and variables. In multiple linear regression analysis, we determined the significant independent factors that could be determinants of BCAA and AAA ratio among the metabolic variables. The serum TG level and weight were found to be independent variables in the normal weight group, and the skeletal muscle mass was an independent variable in the group with obesity.
4. ORs for evaluating metabolic disease according to BCAA and AAA ratio
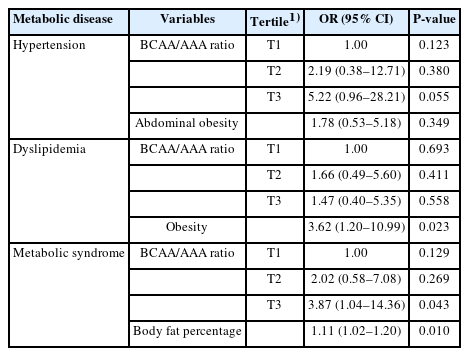
We examined the influence of BCAA and AAA ratio on the risk of metabolic syndrome, hypertension, and dyslipidemia in Table 4. To evaluate the associations of serum BCAA and AAA ratio with obesity and metabolic disease in this study, logistic regression analysis was performed. The relations with hypertension were stronger in the higher tertile when compared with lowest tertile of BCAA and AAA ratio hypertension (OR, 5.22; 95% CI, 0.96–28.21; P= 0.055). The BCAA/AAA ratio showed significant positive associations with metabolic syndrome (OR, 3.87; 95% CI, 1.04–14.36; P= 0.043) in the higher tertile, and body fat percent showed positive associations with metabolic syndrome (OR, 1.11; 95% CI, 1.02–1.20; P= 0.010). There was no significant association between BCAA and AAA ratio and dyslipidemia. But obesity was found to be independent risk factors for dyslipidemia in the logistic regression model (OR, 3.62; 95% CI, 1.20–10.99; P= 0.023).
DISCUSSION
Over the past few decades, there has been a dramatic increase in the prevalence of metabolic diseases and obesity. Two of the major worldwide health problems are obesity and metabolic diseases [21,22]. The obese status emphasizes the need to understand how metabolite profiles are altered. In this study, we determined the relationship between serum AA profiles and metabolic disease risks in the Korean population. Our study showed that concentrations of BCAA and AAA in patients with obesity could identify patients who are at risk for metabolic syndrome and hypertension. And we showed that the BCAA and AAA ratio is useful in assessing metabolic diseases.
The participants were classified based on their BMI into 2 groups, as follows: normal weight group and group with obesity. The serum BCAA, AAA, as well as BCAA and AAA ratio in the group with obesity were significantly higher than those in the normal weight group. Consistently, in a prospective study of middle-aged Japanese workers, significant differences were noted in the associations between serum concentrations of isoleucine, valine, and tyrosine and insulin resistance, particularly between those with and without overweight/obesity. Only the individuals who were obese or overweight showed a significant association [23]. Moreover, in a prospective study of Finnish adults, it was reported that elevated levels of BCAA and AAA were linked to a higher risk of developing insulin resistance over a period of 6 years [14].
Increased serum BCAA and AAA in obese individuals may be related to high-protein diets, altered protein turnover, or altered AA catabolism [24]. High dietary intake of BCAA was linked to an elevated risk of insulin resistance [4]. Thus, our observation of serum BCAA and AAAs could have been owing to the high dietary intake or with insulin resistance among individuals with obesity. BCAA have been shown to stimulate the secretion of both insulin and glucagon, which can lead to increased and prolonged secretion of these hormones [25]. Insulin can increase the uptake of AA, including BCAA, and promote protein synthesis in the body [7,24]. As a result, this leads to an increase in skeletal muscle mass, which can eventually contribute to an increase in overall body weight [26].
We investigated the associations of BCAA and AAA ratios with other metabolite factors in the normal-weight and obesity group. The correlation analyses showed positive associations of BCAA and AAA ratio and body weight, TG, and skeletal muscle mass in multivariate linear regression analysis. Further, we evaluated the association of BCAA and AAA ratio with metabolic disease and found a high level of association, with an OR of 5.22 and 3.87 for BCAA and AAA ratio, in hypertension and metabolic syndrome, respectively.
BCAA consist of about 40% of the free essential AAs in human blood. It has been proposed that the common large neutral amino acid transporter (LAT1) transports BCAA and AAA in competition for into mammalian cells [27]. Thus, higher of BCAA in the participants may have contributed to a significant increase in AAA levels in them [28]. Recently, there has been a growing interest in research the potential effects of BCAA and AAA ratio on chronic disease. However, the clinical studies regarding the roles of BCAA and AAA ratio in chronic disease are inconsistent, which show a positive association or negative association of serum BCAA and AAA ratio according to disease. The low levels of BCAA and AAA ratio was significantly associated with liver disease [29] and heart disease [19]. But the high levels of BCAA and AAA ratio was significantly associated with metabolic disease related to insulin resistance such as hypertension [30], diabetes [31], metabolic syndrome [32].
Although the precise mechanisms for the interaction between AA, obesity, and insulin resistance are unknown, it has been proposed that AA—in particular, BCAA—synergize with hyperlipidemia to accelerate the onset of insulin resistance [33]. The serum BCAA levels are raised the catabolic metabolism in the skeletal muscles and liver, leading to found in higher concentrations of catabolic intermediates propionyl-CoA and succinyl-CoA in the bloodstream [25]. This might accumulate as a result in the accumulation of insufficiently oxidized substrates of fatty acids and glucose, mitochondrial stress, and impaired insulin action [34]. The increase in circulation BCAA levels that follows makes biological reason and suggests the possibility that obesity and BCAA cooperate to increase the risk of insulin resistance. Elevated BCAA and lipid interactions may then cause β-cell malfunction, which will cause the obese, insulin-resistant state to change into type 2 diabetes [35]. The AAA plays a role in the dopaminergic pathway and is most strongly linked to hunger and promotion food-based rewards, impacts serotonergic neurotransmission that control of appetite, body weight and emotions. Therefore, we suggest that the BCAA and AAA ratio may contribute to a better understanding of the pathophysiology of the underlying disease, and the identification of biomarkers for the prevention, early detection, and management of metabolic disease.
Limitations of this study include the following. First, the sample size of this study was small, the study design was cross-sectional, and it was conducted in the Korean population only, which therefore limits the application of our results. Second, we did not include serum insulin resistance results and data associated with the lifestyle factors such as exercise and dietary habit, which may have impacted the serum AA profiles. However, this study’s strength is that it examined the associations between BCAA and AAA ratio, obesity, and obesityrelated diseases using various analytical approaches.
CONCLUSIONS
Our study suggested the usefulness of BCAA and AAA ratio as biomarkers for evaluating the risks of metabolic diseases, including obesity, metabolic syndrome, and hypertension in the Korean population. Future studies would require larger sample size and cutoff value analysis to characterize changes in the serum levels of AA in patients with metabolic disease, as diagnostic biomarkers. Moreover, a clinical trial to evaluate lower BCAA and AAA ratio is important to determine whether this could be a new strategy in the treatment of metabolic diseases, including obesity.
Notes
Conflict of Interest
There are no financial or other issues that might lead to conflict of interest.
Funding
This research was supported by Changwon National University in 2023–2024.
Data Availability
The data that support the findings of this study will be made available upon request to the corresponding author.