Articles
- Page Path
- HOME > Korean J Community Nutr > Volume 28(1); 2023 > Article
- Research Article
- Micronutrients and prevention of cervical pre-cancer in HPV vaccinated women: a cross-sectional study
- Chandrika J Piyathilake, Suguna Badiga, Nongnut Thao, Pauline E Jolly
-
Korean Journal of Community Nutrition 2023;28(1):61-73.
DOI: https://doi.org/10.5720/kjcn.2023.28.1.61
Published online: February 28, 2023

2Research associate, Department of Nutrition Sciences, UAB, Birmingham, AL, USA

3Awardee of the Minority Health Research Training grant, St. Olaf college, Birmingham, AL, USA

4Professor, Department of Epidemiology, UAB, Birmingham, AL, USA

-
Corresponding author:
Pauline E Jolly,Fax: +1-205-934-7049,
Email: piyathic@uab.edu
- 548 Views
- 11 Download
- 2 Crossref
- 0 Scopus
Abstract
Objectives
Prophylactic vaccines against high-risk human papillomaviruses (HR-HPVs) hold promise to prevent the development of higher grade cervical intraepithelial neoplasia (CIN 2+) and cervical cancer (CC) that develop due to HR-HPV genotypes that are included in HPV vaccines, but women will continue to develop CIN 2+ and CC due to HR-HPV genotypes that are not included in the quadrivalent HPV vaccine (qHPV) and 9-valent HPV vaccine (9VHPV). Thus, the current vaccines are likely to decrease but not entirely prevent the development of CIN 2+ or CC. The purpose of the study was to determine the prevalence and determinants of CIN 2+ that develop due to HR-HPVs not included in vaccines.
Methods
Study population consisted of 1476 women tested for 37 HPVs and known to be negative for qHPVs (6/11/16/18, group A, n = 811) or 9VHPVs (6/11/16/18/31/33/45/52/58, group B, n = 331), but positive for other HR-HPVs. Regression models were used to determine the association between plasma concentrations of micronutrients, socio-demographic, lifestyle factors and risk of CIN 2+ due to HR-HPVs that are not included in vaccines.
Results
The prevalence of infections with HPV 31, 33, 35 and 58 that contributed to CIN 2+ differed by race. In group A, African American (AA) women and current smokers were more likely to have CIN 2 (OR = 1.76, P = 0.032 and 1.79, P = 0.016, respectively) while in both groups of A and B, those with higher vitamin B12 were less likely to have similar lesions (OR = 0.62, P = 0.036 and 0.45, P = 0.035, respectively).
Conclusions
We identified vitamin B12 status and smoking as independent modifiable factors and ethnicity as a factor that needs attention to reduce the risk of developing CIN 2+ in the post vaccination era. Continuation of tailored screening programs combined with non-vaccine-based approaches are needed to manage the residual risk of developing HPVrelated CIN 2+ and CC in vaccinated women.
Published online Feb 28, 2023.
https://doi.org/10.5720/kjcn.2023.28.1.61
Micronutrients and prevention of cervical pre-cancer in HPV vaccinated women: a cross-sectional study
Abstract
Objectives
Prophylactic vaccines against high-risk human papillomaviruses (HR-HPVs) hold promise to prevent the development of higher grade cervical intraepithelial neoplasia (CIN 2+) and cervical cancer (CC) that develop due to HR-HPV genotypes that are included in HPV vaccines, but women will continue to develop CIN 2+ and CC due to HR-HPV genotypes that are not included in the quadrivalent HPV vaccine (qHPV) and 9-valent HPV vaccine (9VHPV). Thus, the current vaccines are likely to decrease but not entirely prevent the development of CIN 2+ or CC. The purpose of the study was to determine the prevalence and determinants of CIN 2+ that develop due to HR-HPVs not included in vaccines.
Methods
Study population consisted of 1476 women tested for 37 HPVs and known to be negative for qHPVs (6/11/16/18, group A, n = 811) or 9VHPVs (6/11/16/18/31/33/45/52/58, group B, n = 331), but positive for other HR-HPVs. Regression models were used to determine the association between plasma concentrations of micronutrients, socio-demographic, lifestyle factors and risk of CIN 2+ due to HR-HPVs that are not included in vaccines.
Results
The prevalence of infections with HPV 31, 33, 35 and 58 that contributed to CIN 2+ differed by race. In group A, African American (AA) women and current smokers were more likely to have CIN 2 (OR = 1.76, P = 0.032 and 1.79, P = 0.016, respectively) while in both groups of A and B, those with higher vitamin B12 were less likely to have similar lesions (OR = 0.62, P = 0.036 and 0.45, P = 0.035, respectively).
Conclusions
We identified vitamin B12 status and smoking as independent modifiable factors and ethnicity as a factor that needs attention to reduce the risk of developing CIN 2+ in the post vaccination era. Continuation of tailored screening programs combined with non-vaccine-based approaches are needed to manage the residual risk of developing HPV-related CIN 2+ and CC in vaccinated women.
Introduction
Infection with carcinogenic or high-risk human papillomaviruses (HR-HPVs) is the main causative factor for developing cervical intraepithelial neoplasia (CIN), precursor lesions for cervical cancer (CC). CC is the first or second leading cause of cancer deaths in developing countries compared to 14th in the US [1]. Over 400,000 annual deaths occur due to CC in developing countries compared to approximately 4000/year in the US [2, 3]. In the US, 50% and 10% of CCs occur among never screened and in women who are not screened within the past 5 years, respectively, indicating a clear benefit of an organized CC screening program [4]. However, in the US, approximately 79 million women get infected with HPVs, and every year there is an estimated 14 million new cases of HPV infections [5] and approximately, 412,000 women are diagnosed with CIN annually, with an associated cost of $570 million [6]. Prevention of CIN is not only important because of the cost associated with medical care, but also because treatment of the disease has several reproductive and obstetrical related adverse outcomes [7, 8].
Quadrivalent HPV vaccine (qHPV) against HPV 16 and 18 has the ability to prevent CIN 2+ lesions or CC associated with those HPV genotypes [9, 10]. However, the fact that a substantial proportion of CIN 2+ lesions are caused by HR-HPV types other than HPV 16 or 18 has begun to be appreciated [11]. The inclusion of additional HR-HPV genotypes (31, 33, 45, 52, and 58) in the 9-valent HPV vaccine (9VHPV) is expected to have better CIN preventive effects, but even this vaccine does not include six HR-HPV genotypes (35, 39, 51, 56, 59, 68). Previous studies have shown that these non-vaccine HPV genotypes are associated with CC risk [12]. Our studies have shown that non-vaccine HPV genotype associated CIN 2+ lesions may have similar malignant potential compared to such lesions that are associated with HPV genotypes that are included in vaccines [13]. No previous studies, however, have reported the determinants of CIN 2+ lesions that are unlikely to be preventable by HPV vaccines. We previously reported that among women who tested positive for any one of the 13 HR-HPVs, those with supraphysiologic concentrations of plasma folate (> 19.8 ng/ml) and sufficient plasma vitamin B12 (> 200.6 pg/ml) had 70% reduced risk of developing CIN 2+ due to their known cancer protective functions [14]. Carotenes are thought to play a role in reducing the risk of cancer in the head and neck, another HR-HPV associated cancer [15].
Based on this background, the purpose of this study was to: 1) identify the most prevalent non-vaccine HPV genotypes detected in CIN 2+ lesions (cases) compared to ≤ CIN 1 (non-cases); and 2) determine whether plasma concentrations of folate, vitamin B12 and carotene and socio-demographic, lifestyle related CIN risk factors alter the risk of being diagnosed with nonvaccine HPV related CIN 2+.
Methods
1. Study population
1) HPV and CIN status
The study population consisted of 1,476 women who had not received HPV vaccines who were enrolled between 2004-2011 by two studies funded by the National Cancer Institute (NCI) (R01CA105448 and R01CA102489). The details of the study population, inclusion and exclusion criteria have been previously described [14]. All women were tested for 37 anogenital HPV genotypes [13 HR-HPV genotypes, namely, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 and 24 LR-HPV genotypes, namely, HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39 and CP6108] using Roche Diagnostics Linear array following manufacturer's instructions as reported in our previous studies [14]. Eight hundred and eleven women were negative for HPV genotypes included in the qHPV vaccine, but positive for other HR-HPV genotypes, 31, 33, 35, 39, 45, 51,52, 56, 58, 59, 68 (Group A) and 331 women were negative for HPV genotypes included in the 9VHPV vaccine, but positive for other HR-HPV genotypes, 35, 39, 51, 56, 59, 68 (Group B) and those two groups of women were assumed to have being vaccinated and had 100% efficacy and therefore negative for given vaccine HPV genotypes. Among the 811 women in group A, 178 women were diagnosed with CIN 2+ (CIN 2 [n = 124], CIN 3 [n = 54]) and 633 women were diagnosed with ≤ CIN 1 (normal cervical epithelium [n = 53], HPV cytopathic effect [HCE, n = 89], reactive nuclear enlargement [RNE, n = 76] or CIN 1 [n = 415]). Among the 331 women in group B, 59 were diagnosed with CIN 2+ (CIN 2 [n = 43], CIN 3 [n = 16]) and 272 women were diagnosed with ≤ CIN 1 (normal cervical epithelium [n = 19], HCE [n = 36], RNE [n = 32], CIN 1 [n = 185]. Pelvic examinations and collection of cervical cells and biopsies were carried out following the standard operating protocols of the UAB colposcopy clinic.
2) Socio-demographic variables, lifestyle risk factors and sexual behavioral factors
Information on socio-demographic variables, lifestyle risk factors and sexual behavioral factors were obtained through a study coordinator administered questionnaire.
3) Assessment of micronutrient status
Circulating concentrations of folate, vitamin B12 and total carotene were determined using protocols previously established and validated in the Molecular Epidemiology Laboratory of Piyathilake at UAB [16]. Briefly, plasma folate concentrations were determined using the L. casei microbiological assay, plasma vitamin B12 concentrations using a competitive radio-binding assay (SimulTRAC-SNB; MP Biomedicals), and total carotene by spectrophotometry.
2. Data Analysis
To be clinically relevant, we considered any non-vaccine HR-HPV genotype with a prevalence of at least 10% as a requirement to be included in the analysis provided that this prevalence rate was found in any subgroup of race or CIN status. Women infected with HPV genotypes 31, 33, 35, 39, 45, 51, 52, and 58 met this cut point as types not included in the qHPV. HPV genotypes 35, 39 and 51 met this cut point as types not included in the 9VHPV. Differences between non-cases and cases (i.e. between ≤ CIN 1 and CIN 2 and between ≤ CIN 1 and CIN 3) in Group A and in Group B with regard to demographic variables [age (< 25 vs ≥ 25) , race (African American [AA] vs Caucasian American [CA]), education (< high school education vs ≥ high school education)], lifestyle-related risk factors [current smokers vs non-current smokers], sexual behavioral factors [parity (≥ 1 vs 0 live births), lifetime number of sexual partners (< 2 vs ≥ 2), use of hormonal contraceptives (yes vs no)], plasma concentrations of folate, vitamin B12 and total carotene (categorized based on the lowest tertile of the population < lower tertile and ≥ lower tertile) were assessed by Pearson χ2 tests. Unconditional logistic regression models were used to determine the association between socio-demographic, lifestyle related CIN risk factors, sexual behavioral factors, and plasma concentrations of folate, vitamin B12 and total carotene and the risk of being diagnosed with non-vaccine HPV related CIN 2 or CIN 3. All analyses were performed using JMP® Pro 16, RRID:SCR-014242 (SAS Institute Inc. Cary, NC, USA).
Results
1. Prevalence of specific HR-HPV genotypes and CIN lesions
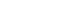
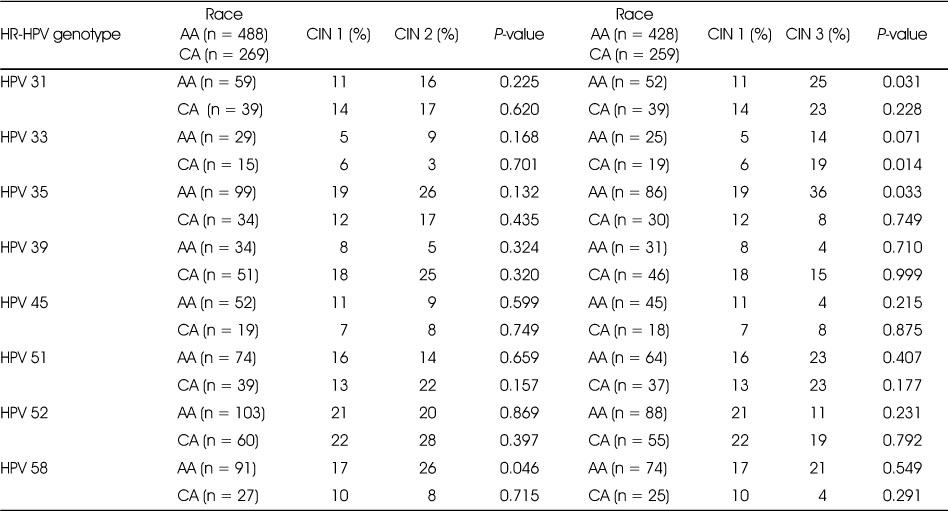
Group A and B comprised of 55% and 22% of the 1476 HR-HPV positive women in our population, respectively. Seventyeight percent of women in group A were non-cases and 22% were cases (15% CIN 2 and 7% CIN 3) and 82% of women in group B were non-cases and 18% were cases (13% CIN 2 and 5% CIN 3). The prevalence of CIN 2 was higher in the AA (17%) compared to the CA (12%) and the prevalence of CIN 3 was higher in CA (9%) compared to the AA (5%) women (P = 0.046). The prevalence of different grades of CIN lesions in group B was similar by race. HR-HPV genotypes with ≥ 10% prevalence in group A were HPV 31, 33, 35, 39, 45, 51 and 52 (Table 1) and in group B, HPV genotypes 35, 39 and 51 (Table 2). We observed that only among AAs, the prevalence of HPV 58 was higher in CIN 2 compared to CIN 1 (17% vs 26%) (P = 0.046) in group A (Table 1) while the prevalence of HPV 35 was higher in CIN 2 compared to CIN 1 (30% vs 67%) (PP < 0.001) in group B. We did not observe a significant difference in the prevalence of HR-HPV genotypes between CIN 1 and CIN 2 in group A or B among CA. HR-HPV genotypes that significantly differed in prevalence between CIN 1 and CIN 3 among AA were HPV 31 (11% vs 25%) (P = 0.031) and 35 (19% vs 36%) (P = 0.033) in group A (Table 1) and HPV 35 (30% vs 75%) (P= 0.013) in group B (Table 2) and among CA HPV 33 (6% vs 19%) (P = 0.014) in group A (Table 1) and none in group B.
Table 1
HR-HPV genotypes not included in qHPV vaccine with ≥ 10% prevalence in at least one lesion category by race in group A1) women
Table 2
HR-HPV genotypes not included in 9vHPV vaccine with ≥ 10% prevalence in at least one lesion category by race in group B1) women
2. Demographic, lifestyle, sexual behavioral factors and micronutrient status of women by lesion diagnoses
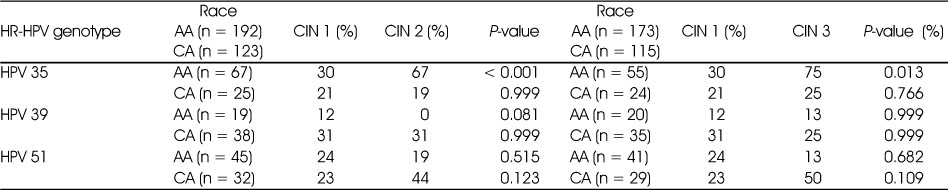
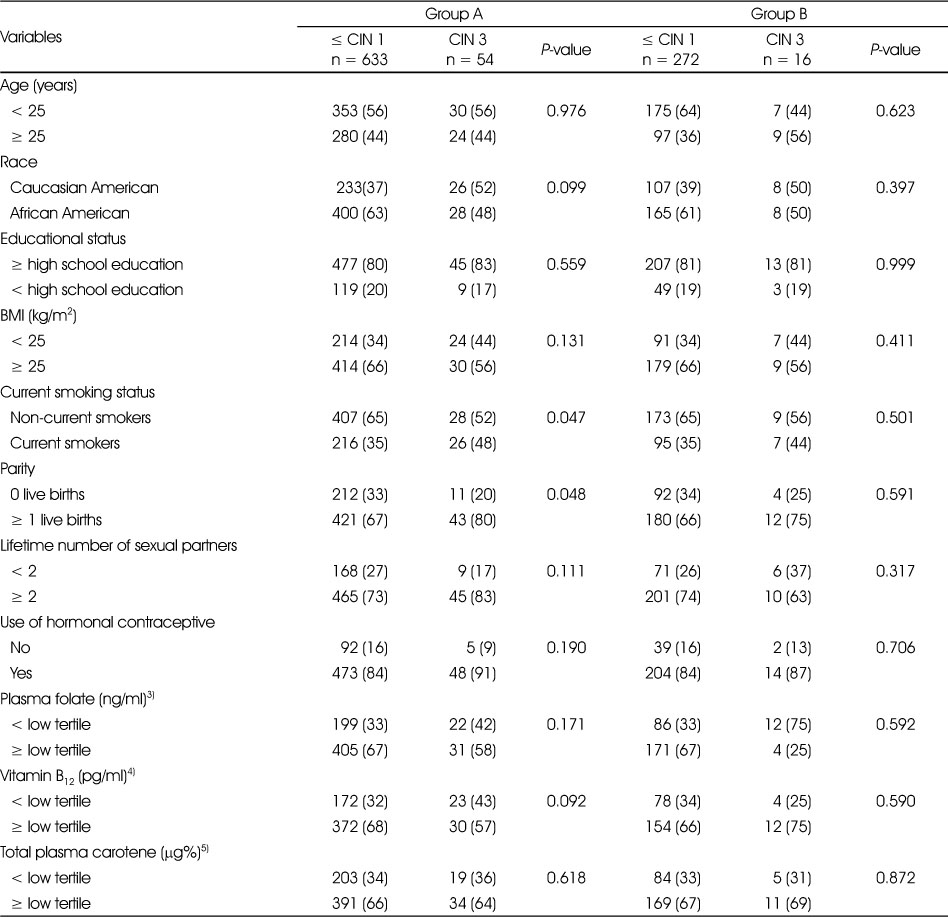
The univariant analysis that tested the differences in the demographic, lifestyle-related risk factors, sexual behavioral factors, and plasma concentrations of folate, vitamin B12 and total carotene between ≤ CIN 1 and CIN 2 in group A and group B are shown in Table 3. In groups A and B, we observed that higher proportions of women diagnosed with CIN 2 compared to women diagnosed with ≤ CIN 1 (85% vs 73% and 88% vs 74%, respectively) had ≥ 2 lifetime number of partners (P = 0.008 and P = 0.039, respectively). In group B, we also observed that a higher proportion of women diagnosed with ≤ CIN 1 compared to women diagnosed with CIN 2 (70% vs 50%) had plasma vitamin B12 concentrations greater than the lower tertile of the population (P = 0.014). None of the other variables significantly differed between ≤ CIN 1 and CIN 2. The univariant analysis that tested the differences in demographic, lifestyle-related risk factors, sexual behavioral factors and plasma concentrations of folate, vitamin B12 and total carotene between ≤ CIN 1 and CIN 3 in group A and group B are shown in Table 4. In group A, a higher proportion of women diagnosed with CIN 3 were current smokers (48% vs 35%) and had a higher number of live births (80% vs 67%) compared to women who were diagnosed with ≤ CIN1 (P = 0.047 and 0.048, respectively). We did not observe any significant differences in other variables between ≤ CIN 1 and CIN 3 in group B.
Table 3
Univariate comparison of demographic, lifestyle, sexual behavioral factors and micronutrient status of women diagnosed with ≤ CIN 1 (non-cases) and CIN 2 (cases) in Group A1) and in Group B2)
Table 4
Univariate comparison of demographic, lifestyle, sexual behavioral factors and micronutrient status of women diagnosed with ≤ CIN 1 (non-cases) and CIN 3 (cases) in Group A1) and in Group B2)
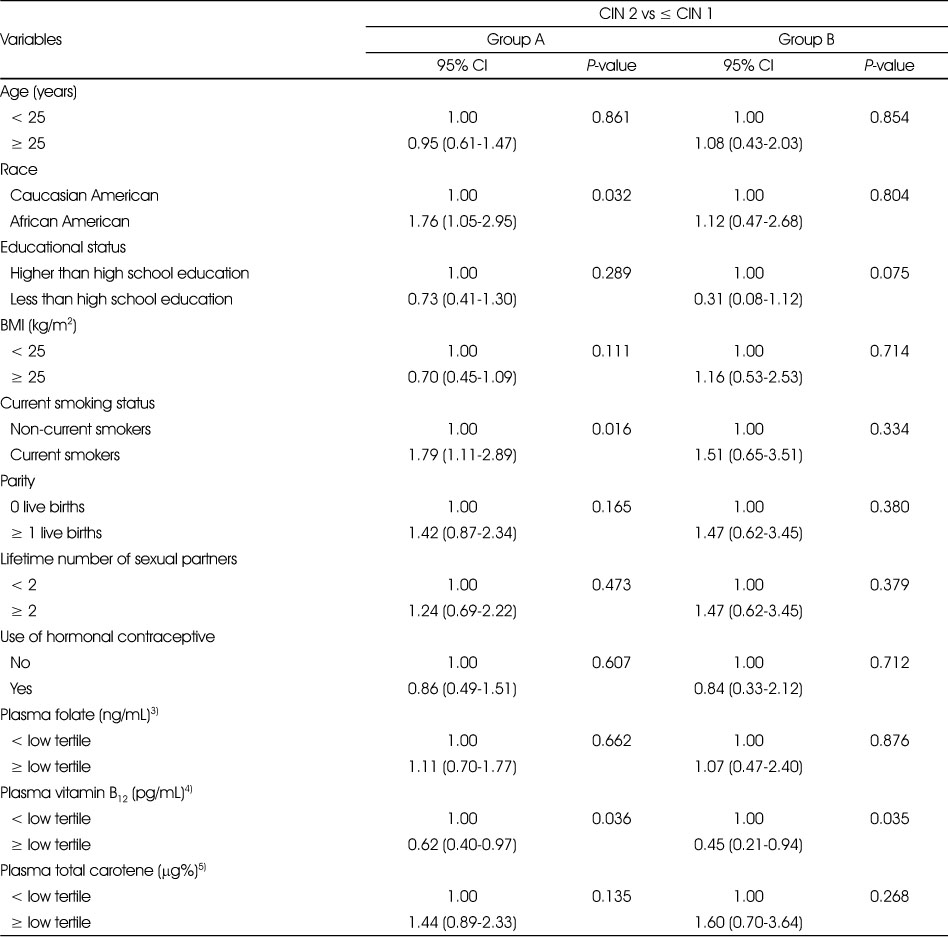
3. Regression model results
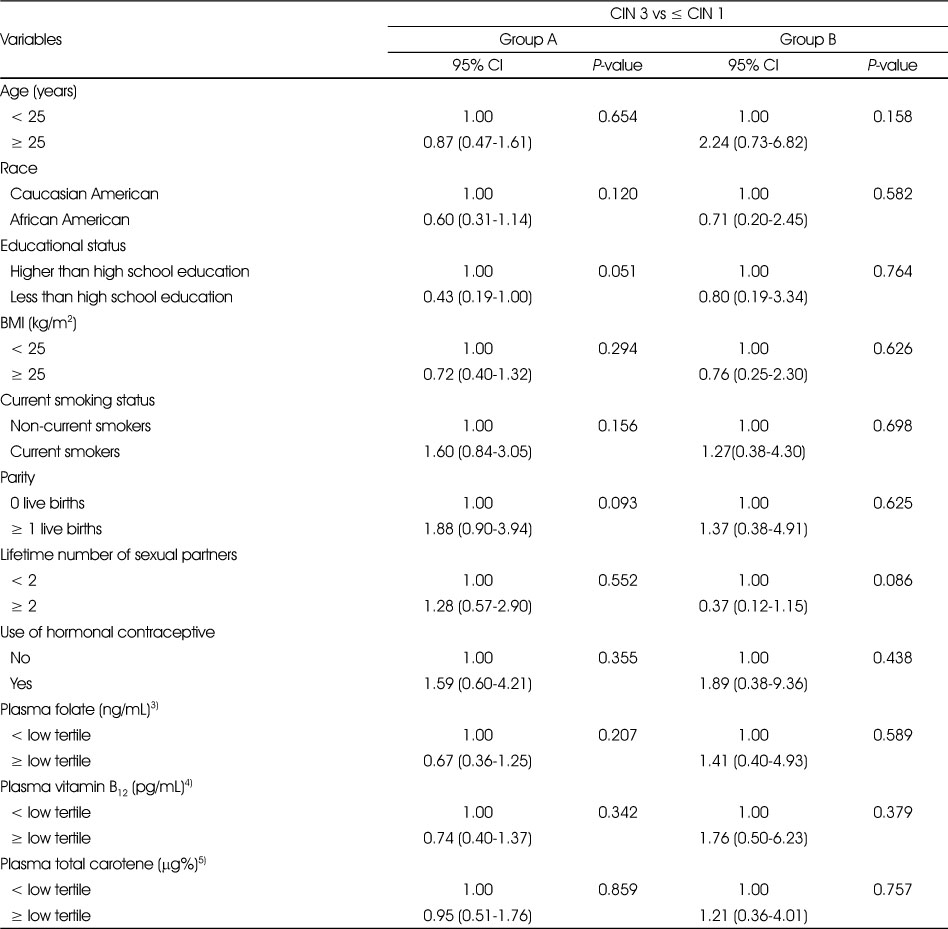
Unconditional logistic regression models that tested the relationships between demographic, lifestyle-related risk factors, sexual behavioral factors and plasma concentrations of folate, vitamin B12 and total carotene and risk of being diagnosed with CIN 2 are shown in Table 5. In group A, we observed that AA women and current smokers were 1.76 and 1.79 times, respectively, were more likely to be diagnosed with CIN 2 compared to CA women and nonsmokers (OR = 1.76, 95% CI = 1.05-2.95, P = 0.032; OR = 1.79, 95% CI = 1.11-2.89, P = 0.016, respectively). In group A, women with plasma vitamin B12 concentration higher than the lower tertile of the population were 38% less likely to be diagnosed with CIN 2 compared to women with plasma vitamin B12 below the lower tertile concentration (OR = 0.62, 95% CI = 0.40-0.97, P = 0.036). In group B, women with plasma vitamin B12 concentrations higher than the lower tertile concentration were 55% less likely to be diagnosed with CIN 2 compared to women with plasma vitamin B12 below the lower tertile concentration (OR = 0.45, 95% CI = 0.21-0.94, P = 0.035). Unconditional logistic regression models that tested the relationships between demographic, lifestyle-related risk factors, sexual behavioral factors and plasma concentrations of folate, vitamin B12 and total carotene and risk of being diagnosed with CIN 3 are shown in Table 6. Although in the univariate analysis, we observed that the proportion of current smokers and women with higher number of live births were significantly different between ≤ CIN 1 and CIN 3, after adjusting for all other variables there were no significant associations between these variables and risk of CIN 3.
Table 5
Relationship between demographic, lifestyle, sexual behavioral factors and micronutrient status and risk of being diagnosed with CIN 2 among group A1) and group B2) women
Table 6
Relationship between demographic, lifestyle, sexual behavioral factors and micronutrient status and risk of being diagnosed with CIN 3 among group A1) and group B2) women
Discussion
Prophylactic HPV vaccines hold promise to prevent the development of CIN 2+ due to HPV genotypes that are included in vaccines, but a demonstrable reduction of the burden of CC, the main goal of prophylactic HPV vaccines, is expected to take several decades. In addition, women will continue to develop CIN 2+ lesions due to HR-HPV genotypes that are not included in such vaccines and those lesions will continue to progress to CC. Therefore, vaccine approach is likely to decrease but not entirely prevent the development of HPV-related CCs for the foreseeable future. Therefore, we envision that a continuation of tailored screening programs combined with lifestyle changes and other non-vaccine-based approaches will be necessary to manage the risk of developing HPV-related cancers in the post-vaccine era. Thirteen HPV genotypes are considered to be high risk, namely, HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. It is generally accepted that HPV 16 and 18 are the most carcinogenic, but studies report that HPV genotypes such as HPV 33, 35 and 45 have similar carcinogenic potential to that of HPV 16 and 18 via producing similar concentrations of mRNA of E6/E7 oncoproteins [17, 18, 19] and those interfere with the normal growth regulatory functions of the host cellular proteins P53 and RB that favor malignant transformation [20, 21, 22]. Even though these observations indicate that women infected with other HR-HPV genotypes, even in the absence of HPV genotypes included in HPV vaccines may develop lesions with similar malignant potential, no studies have identified factors that alter the risk of being diagnosed with non-vaccine HPV related CIN 2+ or the most prevalent non-vaccine HPV genotypes detected in such lesions. Studies in the US and in other countries have demonstrated reductions in CIN 2+ lesions attributable to HR-HPV genotypes targeted by qHPV vaccine [23, 24, 25]. Studies have also estimated that if 9VHPV vaccination programs are effectively implemented, the majority of CIN 2+ due to those HPV genotypes could be prevented [26, 27]. However, the 9VHPV vaccine failed to prevent infection or the development of precancerous lesions in the cervix, vulvar or vagina related to HPV types beyond the nine types covered by this vaccine [31]. Since the magnitude of CIN burden related to non-vaccine HPV genotypes or their determinants is largely unknown, in this study, we addressed these concerns by utilizing a population of women who were are characterized for demographic, lifestyle related CIN risk factors, plasma concentrations of folate, vitamin B12 and carotene, HPV status and histologically confirmed CIN diagnoses. We observed that > 50% of our study population tested positive for HPV genotypes that are not included in qHPV and 15% and 7% of them had CIN 2 or CIN 3, respectively. The percentage of women who tested positive for HR-HPV genotypes that are not included in the 9VHPV vaccine was lower (22%), but 13% and 5% of them had CIN 2 and CIN 3, respectively. These observations indicate that CC risk in women vaccinated with current vaccines cannot be ignored. Further, the cumulative incidence of such lesions could be higher in this population over time since 78% and 82% of women diagnosed with ≤ CIN 1 who are at risk of progressing to CIN 2+ are also positive for HR-HPV genotypes that are not included in qHPV and 9VHPV vaccines, respectively. Further, the prevalence of some of these HPV genotypes such as HPV 35 was higher than 60% and the prevalence was significantly higher in CIN 2 or 3 compared to CIN 1 lesions, especially in AA women. Since AAs and CAs are the most prevalent racial groups in the USA and there are cultural differences with regard to dietary factors between these racial groups, it is important to document racial differences in CIN 2+ risk related to specific HPV genotypes. We observed that women who receive qHPV or 9VHPV vaccines are more likely to develop CIN 2 rather than CIN 3. Even though the severity is lower, CIN 2 lesions left under observation without treatment according to the current American College of Obstetricians and Gynecologists (ACOG) guidelines in women < 25 years of age may transform to CIN 3 by the time they reached screening age. Treatment of CIN 2 in older women results in adverse reproductive and gynecological outcomes indicating the importance of their prevention in vaccinated women, irrespective of their age. We observed that improving vitamin B12 status or its supplementation could serve as a non-vaccine-based approach to reduce the risk of developing such lesions. In contrast to our previous findings reported with women enrolled during at the beginning of the US folic acid fortification period (1996-1998), we did not observe a significant association between lower folate status and higher risk of CIN 2+ [28]. This could be due to the fact that plasma folate concentrations had continued to increase in US over time due to the folate fortification program. We previously reported that in a cohort of women enrolled after 2004, a few years of exposure to folate fortified food, having sufficient vitamin B12 plays a significant role in altering the risk of developing CIN 2+ [14]. Since there was no vitamin B12 fortification program in the US, approximately, one third of women who are at risk for CIN 2+ have inadequate concentrations of vitamin B12 (< 350 pg/mL). In developing countries such as India, however, both folate and vitamin B12 could play a role in reducing CIN 2+ risk as only 2% achieve supra-physiologic concentrations of folate and 66% of women have inadequate vitamin B12 concentrations [29]. These observations suggest that improving the status of both folate and vitamin B12 by dietary means or their supplementation could serve as an effective non-vaccine-based approach to reduce the risk of developing such lesions. Mechanistically, folate is likely to be associated with a lower risk of CIN 2+ in several ways. Folate deficiency leads to DNA instability (DNA and chromosomal damage), risk factors for cancer, by several mechanisms including uracil misincorporation, impaired DNA excision repair, and suboptimal cellular DNA repair capacity [30. 31]. Higher folate minimizes these DNA and chromosomal changes [32]. Intervention studies in humans taking folate supplements have shown that DNA damage in peripheral blood cells is minimized when plasma concentrations of folate is high [30]. Further, DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro [29]. Folate deficiency also modulates DNA repair, DNA strand breakage, and uracil misincorporation in immortalized human colonocytes [33]. Folate’s influence on HPV integration is also a possible explanation since common chromosomal fragile sites that are sensitive to folate deficiency have been shown to coincide with sites of HPV integration, a key event in HPV carcinogenesis [34]. Further, our previous studies have showed that a lower degree of HPV methylation in the presence of lower plasma concentrations of folate and vitamin B12 influence the biology of specific HPV genotypes and the likelihood of being diagnosed with CIN 2+ [35], indicating the importance of both folate and vitamin B12 to prevent the development of CIN 2+. We also observed that AA women and smokers who will be given qHPV vaccine may need regular follow up and advice on smoking cessation suggesting that tailored preventive measures based on ethnicity and adverse health habits are important. In this study, we noted that none of the known risk factors evaluated was significantly associated with the risk of developing CIN 3. However, since the magnitude of the association was similar for some risk factors such as smoking, the smaller sample size may explain these non-significant results. It is also possible that other factors that are not examined such as methylation of HPV or their integration status could be contributing to the development of such CIN lesions. Collectively, our results demonstrated the importance of continuation of tailored screening programs combined with lifestyle changes/other non-vaccines-based approaches to manage the residual risk of developing HPV related cancers in the post-HPV vaccination era as the prevalence of some HPV genotypes that are not included are frequently found in this population. Further, since the incidence of HPV-associated pre-cancers and cancers also continues to increase at several anatomical sites other than the cervix (oropharynx, anus, vagina, vulva) [36] in the post-HPV vaccination era, it is important to study and extend similar preventive approaches to control the risk of developing cancers in those organs. Our study approach will form the foundation for such studies. In this study, we assumed that women who tested negative for qHPV or 9VHPV genotypes could have received those vaccines and had 100% efficacy in preventing infections with those vaccine HPV genotypes. Even though this assumption can be viewed as a limitation, it is important to acknowledge that HPV vaccine studies are in general are conducted in younger females who are naive to HPV infections and therefore cervical cells to test for HPV infections or biopsies to evaluate CIN diagnoses are rarely collected in these studies and therefore it is not possible to test the aims of our study in those populations. Our approach will form the foundation to design post vaccination era studies in the future to test the significance of tailored screening programs combined with lifestyle changes/other non-vaccines-based approaches to manage the residual risk of developing HPV related cancers in vaccinated women. Since 2016, only 9VHPV vaccine is used in the US. However, there is no Advisory Committee on Immunization Practices (ACIP) recommendation regarding additional 9VHPV vaccine doses for people who have completed the vaccine series with the qHPV vaccine other than 9VHPV vaccine may be used to continue or complete a vaccination series started with qHPV vaccine [37, 38, 39, 40]. Therefore, both groups of women, negative for qHPV and 9V HPV genotypes will exist in the US post HPV vaccination era. Since in developing countries only the qHPV vaccine is used currently, vaccinated women will only be negative for qHPV genotypes.
Conclusions
The current HPV vaccine approach is likely to decrease but not prevent the development of cervical precancerous lesions entirely in a significant proportion of women. Continuation of screening programs combined with lifestyle changes and other non-vaccine-based approaches are needed to manage the risk of developing HPV-related cancers in vaccinated women. We identify vitamin B12 status and smoking as independent modifiable factors and ethnicity as a factor that needs attention to reduce the risk of developing CIN 2+ in the post vaccination era. Larger studies are needed to confirm the significance of other factors such as educational status, parity and the number of lifetime partners that reached borderline significance in relation to CIN 2 or 3 in our study. Since the current study is a cross-sectional study where risk factors and plasma concentrations micronutrients were assessed at a single time point, the temporality of the associations or causal relations cannot be assessed in our study. Verification of our results in longitudinal studies and their reproducibility in other populations exposed to HPV infections and/ vaccines will increase the scientific validity of our findings.
Ethics statement:The Institutional Review Board at the UAB approved all study protocols in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All women had given their informed consent prior to their inclusion in the study (IRB approval number 040126002).
Conflict of Interest:The authors declare no potential conflicts of interest.
Funding:Supported by R01 105448 (National Cancer Institute) and T37-MD001448 (Minority Health Research Training grant, National Institute on Minority Health and Health Disparities).
Data availability
Data described in the manuscript will be made available upon request and approval by the corresponding author.
References
-
HPV Information Centre. Human Papillomavirus and related cancers, Fact sheet 2021 [Internet]. HPV Information Centre; 2021 [cited 2022 Jul 4].Available from: https://hpvcentre.net/statistics/reports/USA_
FS.pdf .
-
-
American Cancer Society. Key statistics for cervical cancer [Internet]. American Cancer Society; 2019 [cited 2022 Jul 4].Available from: https://www.cancer.org/cancer/cervical-
cancer/about/key- statistics.html .
-
-
HPV Information Centre. Statistics [Internet]. HPV Information Centre; 2021 [cited 2022 Jul 4].Available from: http://www.hpvcentre.net/datastatistics.php .
-
-
Center for Disease Control and Prevention. Report to Congress: Prevention of Genital Human Papillomavirus Infection [Internet]. Center for Disease Control and Prevention; 2004 [cited 2022 Jul 25].Available from: https://www.cdc.gov/std/hpv/2004hpv%20report.pdf .
-
-
Centers for Disease Control and Prevention. Genital HPV infection-CDC Fact sheet [Internet]. Centers for Disease Control and Prevention; 2017 [cited 2022 Sep 8].Available from: https://www.cdc.gov/std/hpv/HPV-
FS- July- 2017.pdf .
-
-
Ferreira SE. Cervical intraepithelial neoplasia diagnosis: Emotional impact and nursing implications. Clin Excell Nurse Pract 1998;2(4):218–224.
-
-
Brewczyński A, Jabłońska B, Kentnowski M, Mrowiec S, Składowski S, Rutkowski T. The association between carotenoids and head and neck cancer risk. Nutrients 2021;14(1):88
-
-
Piyathilake CJ, Badiga S, Paul P, Vijayaraghavan K, Vedantham H, Sudula M, et al. Indian women with higher serum concentrations of folate and vitamin B12 are significantly less likely to be infected with carcinogenic or high-risk (HR) types of human papillomaviruses (HPVs). Int J Womens Health 2010;2:7–12.
-
-
Chaturvedi AK. Beyond cervical cancer: Burden of other HPV-related cancers among men and women. J Adolesc Health 2010;46(4):S20–S26.
-
-
Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300–304.
-
-
Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63(5):1–30.
-
-
Centers for Disease Control and Prevention. Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV [Internet]. Centers for Disease Control and Prevention; 2016 [cited 2022 Sep 1].Available from: https://www.cdc.gov/hpv/downloads/9vhpv-
guidance.pdf .
-
-
Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination-updated recommendations of the advisory committee on immunization practices. Am J Transplant 2017;17(3):834–837.
-

 KSCN
KSCN

 Cite
Cite