Articles
- Page Path
- HOME > Korean J Community Nutr > Volume 28(3); 2023 > Article
-
Review
- Mercury exposure is associated with obesity: a systematic review and meta-analysis
-
Jimin Jeon1)
 , Kyong Park2),†
, Kyong Park2),†
-
Korean Journal of Community Nutrition 2023;28(3):192-205.
DOI: https://doi.org/10.5720/kjcn.2023.28.3.192
Published online: June 30, 2023
1)Researcher, Department of Neurology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
2)Professor, Department of Food and Nutrition, Yeungnam University, Gyeongsan, Korea
- †Corresponding author Kyong Park Department of Food and Nutrition, Yeungnam University, 280 Daehak-ro, Gyeongsan, Gyeongbuk 38541, Korea Tel: +82-53-810-2879 Fax: +82-53-810-4666 E-mail: kypark@ynu.ac.kr
© 2023 The Korean Society of Community Nutrition
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,638 Views
- 71 Download
- 4 Crossref
Abstract
-
Objectives
- Previous studies have evaluated the association between mercury exposure and obesity but have yielded mixed conclusions. The aim of this study was to systematically review and summarize scientific evidence regarding the association between mercury exposure and obesity in the human population.
-
Methods
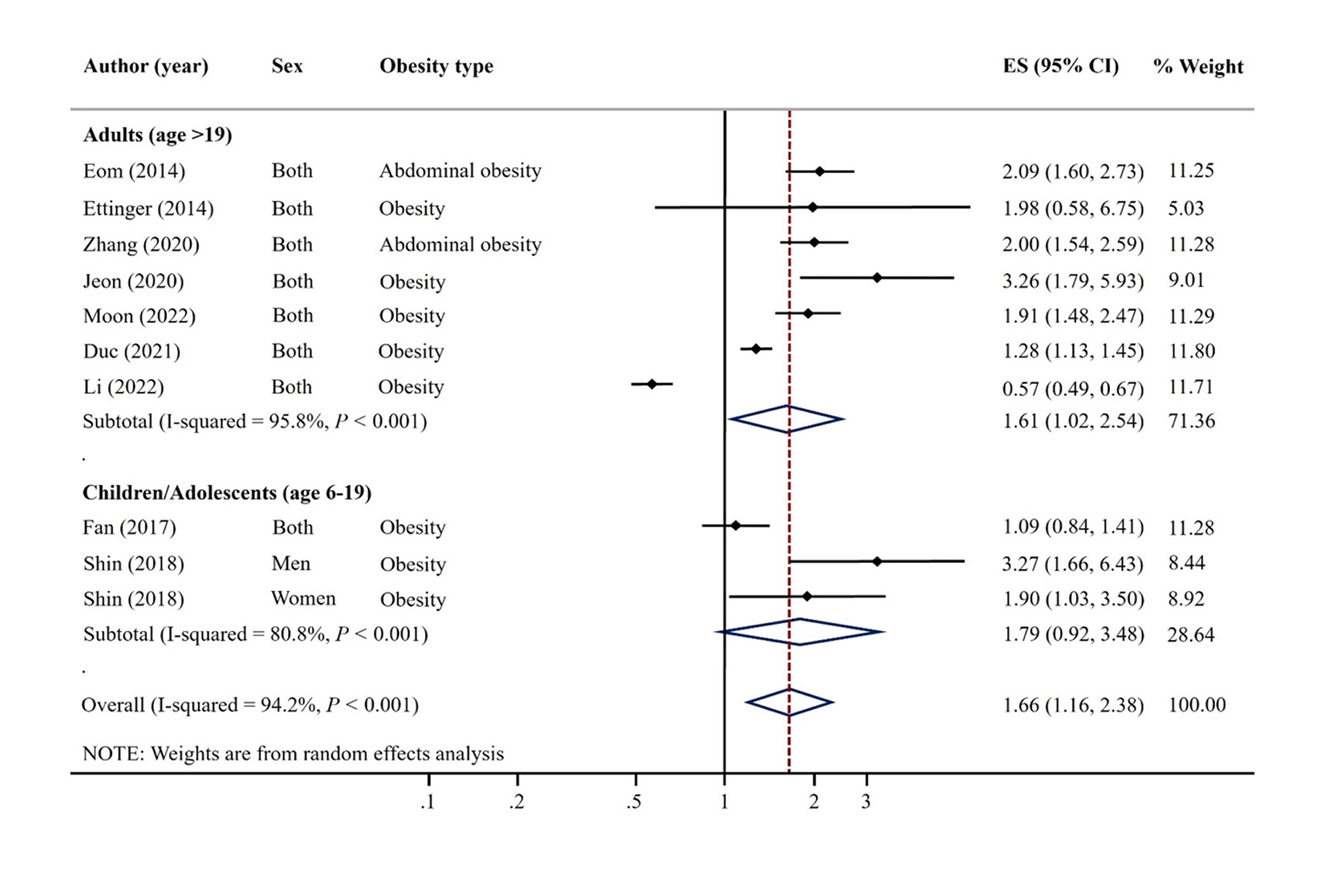
- We conducted a systematic search of PubMed, Web of Science, Scopus, and Science Direct for articles related to mercury exposure and obesity. Meta-analyses of the highest and lowest categories of mercury levels were evaluated using a random effects model. Begg’s test was used to detect publication bias.
-
Results
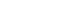
- A total of 9 articles were included. The pooled random effects odds ratio (OR) for mercury exposure and obesity of all 9 studies was 1.66 (95% confidence interval [CI]: 1.16-2.38). This positive association was evident in adults (OR: 1.61, 95% CI: 1.02-2.54) and among studies with Asian populations (OR: 2.00, 95% CI: 1.53-2.59), but not among those with North America/African populations (OR: 0.90, 95% CI: 0.50-1.65).
-
Conclusions
- The present meta-analysis identified a positive association between mercury exposure and obesity. These findings suggest that toxic environmental metals such as mercury may be an important risk factor for obesity along with dietary habits and lifestyles.
Introduction
Methods
Results
Discussion
Conclusion
-
Conflict of Interest
As the Editor-in-Chief of the Korean Journal of Community Nutrition, I, Kyong Park, declare a potential conflict of interest with this publication. I ensured neutrality by abstaining from the manuscript's review process, managed by an independent associate editor. Otherwise, there are no financial or other issues that might lead to a conflict of interest.
-
Funding
This research was supported by the National Research Foundation of Korea grant funded by the Korea government (grant number: NRF-2021R1A2C1007869). The funding sponsor had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the article for publication.
-
Acknowledgments
The authors thank Jihyun Im, Unhui Jo, Chaehyun Kim, Hyeonji Yoo, and Yeeun Park for their technical contributions to this work.
-
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Fig. 1.


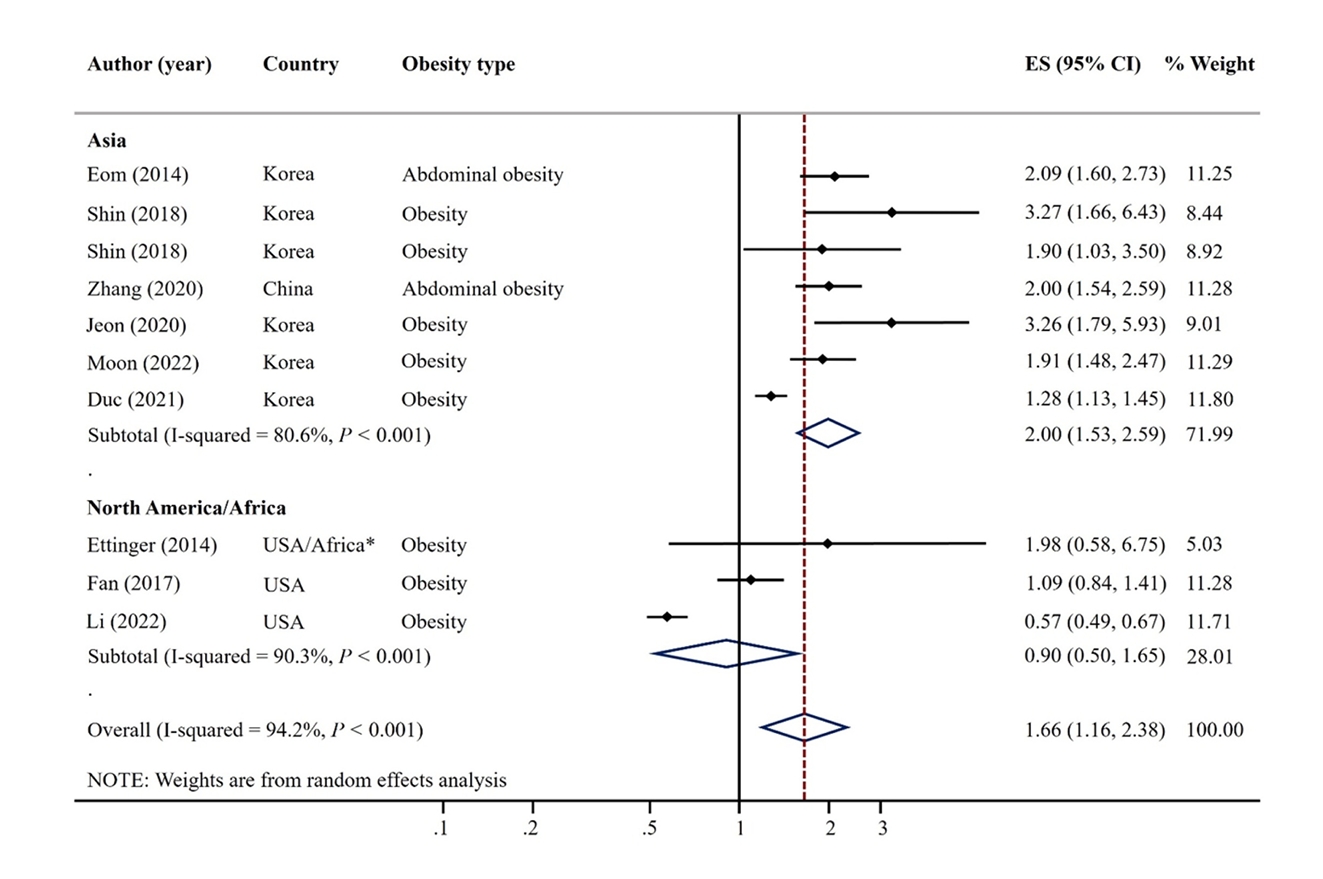
| Author (year) | Country | Design | Sample size | Participant/Study name | Recruit period (year) | Sex | Mean or range of age (years) | Exposure | Outcome | Obesity definition | OR/PR (95% CI) | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eom (2014) [14] | Korea | Cross-sectional | 2,114 | Adults from metropolitan and rural districts | 2010-2011 | M | 45.5 | Blood mercury (μg/L) | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.09 (1.60, 2.72) | Sex, age, smoking, alcohol drinking, household income, residence area, seafood intake |
| W | ||||||||||||
| Ettinger (2014) [24] | Ghana, South Africa, Seychelles, Jamaica, USA | Cross-sectional | 150 | METS | 2010-2011 | M | 25-45 | Blood mercury (μg/L) | Obesity | BMI ≥ 25 kg/m2 | 1.98 (0.58, 6.74) | Sex, age, residence area, marital status, education level, paid employment, smoking, alcohol drinking, fish intake |
| W | Abdominal obesity | WC ≥ 94 cm for men, ≥ 80 cm for women | 1.47 (0.43, 5.00) | |||||||||
| Fan (2017) [26] | USA | Cross-sectional | 5,404 | NHANES | 2011-2014 | M | 6-19 | Blood mercury (μg/L) | Obesity | BMI percentile | 1.09 (0.84, 1.41) | Sex, age, race, poverty income ratio, TV, computer, and video games use in hours, BMI |
| W | ||||||||||||
| Shin (2018) [15] | Korea | Cross-sectional | 1,567 (M 793 W 774) | KNHANES | 2010-2013 | M | 15.0 | Blood mercury (μg/L) | Overweight/obesity | 1) BMI ≥ 85th percentile (aged < 19 years) | M 3.27 (1.66, 6.41) | Age, household income, energy intake, fulfillment of moderateto-vigorous physical activity |
| 2) BMI ≥ 23 kg/m2 (aged 19 years) | W 1.90 (1.03, 3.49) | |||||||||||
| W | Abdominal obesity | WHR ≥ 0.5 | M 2.35 (1.05, 5.24) | |||||||||
| W 1.67 (0.57, 4.93) | ||||||||||||
| Zhang (2020) [19] | China | Case-control | 4,134 | Beijing Population Health Cohort study | 2017 | M | 50-75 | Blood mercury (μg/L) | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.00 (1.55, 2.60) | Education level, smoking, alcohol drinking, BMI, physical activity, family history of disease |
| W | ||||||||||||
| Jeon (2021) [16] | Korea | Cross-sectional | 495 | SELEN | 2012-2013 | M | 40-69 | Toenail mercury (μg/g) | Obesity | BMI ≥ 25 kg/m2 | 3.26 (1.79, 5.93) | Age, sex, education level, residential area, monthly household income, smoking status, alcohol consumption, physical activity, total energy intake, use of dietary supplements |
| W | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.30 (1.15, 4.59) | |||||||||
| Moon (2022) [17] | Korea | Cross-sectional | 3,787 | KoNHES | 2015-2017 | M | ≥ 19 | Blood mercury (μg/L) | Obesity | BMI ≥ 25 kg/m2 | 1.91 (1.48, 2.47) | Age, sex, smoking, alcohol drinking, exercise, education levels |
| Duc (2021) [18] | Korea | Cross-sectional | 6,434 | KNHANES | 2009-2017 | M | ≥ 50 | Blood mercury (μg/L) | Obesity | BMI ≥ 27.5 kg/m2 | 1.28 (1.13, 1.45) | Comorbidities, sex, age, energy intake, occupation, family history of hyperlipidemia, family history of CVD, physical activity, drinking status, residential areas, smoking, ln2 cotinine, educational level, and monthly household incomes |
| W | Abdominal obesity | WC ≥ 90 cm for men or ≥ 80 cm for women | 1.24 (1.13, 1.36) | |||||||||
| Li (2022) [25] | USA | Cross-sectional | 15,959 | NHANES | 2007-2018 | M | ≥ 20 | Blood mercury (μg/L) | Obesity | BMI ≥ 30 kg/m2 | 0.57 (0.49, 0.67) | Sex, age, race, income, education, marriage, smoking, drinking status, physical activity, diabetes, cardiovascular disease |
| W | Abdominal obesity | WC ≥ 102 cm for men or ≥ 88 cm for women | 0.56 (0.49, 0.65) |
OR, odds ratio; PR, prevalence ratio; CI, confidence interval; M, men; W, women; KNHANES, Korea National Health and Nutrition Examination Survey; KoNHES, Korean National Environmental Health Survey; NHANES, National Health and Nutrition Examination Survey; METS, Modeling the Epidemiologic Transition Study; SELEN, Trace Element Study of Korean Adults in the Yeungnam area
- 1. Martinez-Finley EJ, Aschner M. Recent advances in mercury research. Curr Environ Health Rep 2014; 1(2): 163-171.ArticlePubMedPMCPDF
- 2. Risher JF; World Health Organization. Elemental mercury and inorganic mercury compounds: Human health aspects. Geneva, Switzerland: World Health Organization; 2003. p. 7-9.
- 3. Bernhoft RA. Mercury toxicity and treatment: A review of the literature. J Environ Public Health 2012; 2012.ArticlePDF
- 4. Fernandes Azevedo B, Barros Furieri L, Pecanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simoes M, et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol 2012; 2012: 949048.PubMedPMC
- 5. Park YK, Seok KJ, Lee SH, Jang SC, Jung WS, Lee JM. Association between GFR and hair mercury, lead level. Korean J Fam Med 2015; 5(3): 961-965.
- 6. Duruibe JO, Ogwuegbu M, Egwurugwu J. Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2007; 2(5): 112-118.
- 7. He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: The CARDIA Trace Element Study. Diabetes Care 2013; 36(6): 1584-1589.PubMedPMC
- 8. Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 2002; 347(22): 1747-1754.ArticlePubMed
- 9. Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995; 91(3): 645-655.ArticlePubMed
- 10. Hu XF, Singh K, Chan HM. Mercury exposure, blood pressure, and hypertension: A systematic review and dose-response meta-analysis. Environ Health Perspect 2018; 126(7): 076002.ArticlePubMedPMC
- 11. Lee S, Yoon JH, Won JU, Lee W, Lee JH, Seok H, et al. The association between blood mercury levels and risk for overweight in a general adult population: Results from the Korean National Health and Nutrition Examination Survey. Biol Trace Elem Res 2016; 171(2): 251-261.ArticlePubMedPDF
- 12. Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J Hazard Mater 2011; 185(2-3): 749-755.ArticlePubMed
- 13. Bjerregaard P, Pedersen HS, Mulvad G. The associations of a marine diet with plasma lipids, blood glucose, blood pressure and obesity among the inuit in Greenland. Eur J Clin Nutr 2000; 54(9): 732-737.ArticlePubMedPDF
- 14. Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health 2014; 87(5): 501-513.ArticlePubMedPDF
- 15. Shin YY, Ryu IK, Park MJ, Kim SH. The association of total blood mercury levels and overweight among Korean adolescents: Analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) 2010-2013. Korean J Pediatr 2018; 61(4): 121-128.ArticlePubMedPMCPDF
- 16. Jeon J, Morris JS, Park K. Toenail mercury levels positively correlate with obesity and abdominal obesity among Korean adults. J Trace Elem Med Biol 2021; 64: 126678.ArticlePubMed
- 17. Moon MK, Lee I, Lee A, Park H, Kim MJ, Kim S, et al. Lead, mercury, and cadmium exposures are associated with obesity but not with diabetes mellitus: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ Res 2022; 204(Pt A): 111888.ArticlePubMed
- 18. Duc HN, Oh H, Kim MS. The effect of mixture of heavy metals on obesity in individuals ≥50 years of age. Biol Trace Elem Res 2021; 200(8): 3554-3571.ArticlePubMedPDF
- 19. Zhang W, Du J, Li H, Yang Y, Cai C, Gao Q, et al. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ Int 2020; 143: 105959.ArticlePubMed
- 20. Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009; 121(6): 21-33.ArticlePubMedPMC
- 21. Zhu X, Kusaka Y, Sato K, Zhang Q. The endocrine disruptive effects of mercury. Environ Health Prev Med 2000; 4(4): 174-183.ArticlePubMedPMCPDF
- 22. Hoffman DJ, Heinz GH. Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem 1998; 17(2): 161-166.Article
- 23. Rizzetti DA, Corrales P, Piagette JT, Uranga-Ocio JA, Medina-Gomez G, Pecanha FM, et al. Chronic mercury at low doses impairs white adipose tissue plasticity. Toxicology 2019; 418: 41-50.ArticlePubMed
- 24. Ettinger AS, Bovet P, Plange-Rhule J, Forrester TE, Lambert EV, Lupoli N, et al. Distribution of metals exposure and associations with cardiometabolic risk factors in the “Modeling the Epidemiologic Transition Study”. Environ Health 2014; 13: 90.ArticlePubMedPMCPDF
- 25. Li T, Yu L, Yang Z, Shen P, Lin H, Shui L, et al. Associations of diet quality and heavy metals with obesity in adults: A cross-sectional study from National Health and Nutrition Examination Survey (NHANES). Nutrients 2022; 14(19): 4038.ArticlePubMedPMC
- 26. Fan Y, Zhang C, Bu J. Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 2017; 9(2): 104.ArticlePubMedPMC
- 27. Bulka CM, Persky VW, Daviglus ML, Durazo-Arvizu RA, Argos M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011-2014. Environ Res 2019; 168: 397-405.ArticlePubMedPMC
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009; 151(4): 264-269.ArticlePubMedPDF
- 29. National Heart Lung and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies [internet]. National Heart Lung and Blood Institute; 2013 [cited 2022 Sep 15]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessmenttools
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557-560.ArticlePubMedPMC
- 31. Trayhurn P, Beattie JH. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60(3): 329-339.ArticlePubMed
- 32. Trayhurn P. Endocrine and signalling role of adipose tissue: New perspectives on fat. Acta Physiol Scand 2005; 184(4): 285-293.ArticlePubMed
- 33. Barnes DM, Hanlon PR, Kircher EA. Effects of inorganic HgCl2 on adipogenesis. Toxicol Sci 2003; 75(2): 368-377.ArticlePubMed
- 34. Kawakami T, Hanao N, Nishiyama K, Kadota Y, Inoue M, Sato M, et al. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol Appl Pharmacol 2012; 258(1): 32-42.ArticlePubMed
- 35. Havel PJ. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc Nutr Soc 2000; 59(3): 359-371.ArticlePubMed
- 36. Berger JP, Akiyama TE, Meinke PT. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci 2005; 26(5): 244-251.ArticlePubMed
- 37. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 2009; 12(3): 206-223.ArticlePubMed
- 38. Vachhrajani KD, Chowdhury AR. Distribution of mercury and evaluation of testicular steroidogenesis in mercuric chloride and methylmercury administered rats. Indian J Exp Biol 1990; 28(8): 746-751.PubMed
- 39. Davis BJ, Price HC, O'Connor RW, Fernando R, Rowland AS, Morgan DL. Mercury vapor and female reproductive toxicity. Toxicol Sci 2001; 59(2): 291-296.ArticlePubMed
- 40. Lee K. Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011-2013 data. Int Arch Occup Environ Health 2018; 91(2): 185-193.ArticlePubMedPDF
- 41. United Nations Environment Programme. Global Mercury Assessment 2018 [internet]. United Nations Environment Programme; 2018 [cited 2022 Feb 6]. Available from: https://www.unenvironment.org/resources/publication/global-mercury-assessment-2018
- 42. Ruggieri F, Majorani C, Domanico F, Alimonti A. Mercury in children: Current state on exposure through human biomonitoring studies. Int J Environ Res Public Health 2017; 14(5): 519.ArticlePubMedPMC
- 43. Calabrese EJ, Baldwin LA. U-shaped dose-responses in biology, toxicology, and public health. Annu Rev Public Health 2001; 22: 15-33.ArticlePubMed
- 44. You CH, Kim BG, Kim YM, Lee SA, Kim RB, Seo JW, et al. Relationship between dietary mercury intake and blood mercury level in Korea. J Korean Med Sci 2014; 29(2): 176-182.ArticlePubMedPMCPDF
- 45. Zillioux EJ. Mercury in fish: History, sources, pathways, effects, and indicator usage. In: Armon RH, Hänninen O, editors. Environmental Indicators. Berlin: Springer; 2015. p. 743-766.
- 46. Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2016 [internet]. Food and Agriculture Organization of the United Nations; 2016 [cited 2022 Oct 18]. Available from: http://www.fao.org/documents/card/en/c/2c8bcf47-2214-4aeb-95b0-62ddef8a982a/
- 47. European Market Observatory for Fisheries and Aquaculture Products. The EU fish market: 2018 edition [internet]. European Market Observatory for Fisheries and Aquaculture Products; 2018 [cited 2022 Nov 14]. Available from: https://www.eumofa.eu/market-analysis# yearly
- 48. Park K. Population correlates of circulating mercury levels in Korean adults: The Korea National Health and Nutrition Examination Survey IV. BMC Public Health 2014; 14: 527.PubMedPMC
- 49. Tinggi U. Selenium: Its role as antioxidant in human health. Environ Health Prev Med 2008; 13(2): 102-108.ArticlePubMedPMCPDF
- 50. Berry MJ, Ralston NV. Mercury toxicity and the mitigating role of selenium. EcoHealth 2008; 5(4): 456-459.ArticlePubMedPDF
- 51. Seppanen K, Kantola M, Laatikainen R, Nyyssonen K, Valkonen VP, Kaarlopp V, et al. Effect of supplementation with organic selenium on mercury status as measured by mercury in pubic hair. J Trace Elem Med Biol 2000; 14(2): 84-87.ArticlePubMed
- 52. Park K, Seo E. Association between toenail mercury and metabolic syndrome is modified by selenium. Nutrients 2016; 8(7): 424.ArticlePubMedPMC
- 53. Park K, Seo E. Toenail mercury and dyslipidemia: Interaction with selenium. J Trace Elem Med Biol 2017; 39: 43-49.ArticlePubMed
- 54. Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 2005; 25(1): 228-233.ArticlePubMed
- 55. Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, et al. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med 2011; 364(12): 1116-1125.ArticlePubMedPMC
- 56. Combs GF. Selenium in global food systems. Br J Nutr 2001; 85(5): 517-547.ArticlePubMed
- 57. Choi YS, Hesketh JE. Nutritional biochemistry of selenium. J Korean Soc Food Sci Nutr 2006; 35(5): 661-670.Article
- 58. Xing K, Zhou S, Wu X, Zhu Y, Kong J, Shao T, et al. Concentrations and characteristics of selenium in soil samples from Dashan Region, a selenium-enriched area in China. Soil Sci Plant Nutr 2015; 61(6): 889-897.Article
- 59. Holland HD, Lollar BS, Turekian KK. Environmental geochemistry. Oxford, UK: Elsevier; 2005. p. 46-48.
- 60. Kim YJ, Galindev O, Sei JH, Bae SM, Im H, Wen L, et al. Serum selenium level in healthy Koreans. Biol Trace Elem Res 2009; 131(2): 103-109.ArticlePubMedPDF
REFERENCES
Figure & Data
REFERENCES
Citations

- Re-thinking the link between exposure to mercury and blood pressure
Xue Feng Hu, Allison Loan, Hing Man Chan
Archives of Toxicology.2025; 99(2): 481. CrossRef - Associations of Metal Mixtures During Early Pregnancy With Midlife Obesity and Body Composition: A Prospective Study
Mingyu Zhang, Izzuddin M. Aris, Andres Cardenas, Sheryl L. Rifas‐Shiman, Pi‐I Debby Lin, Long H. Ngo, Emily Oken, Stephen P. Juraschek, Marie‐France Hivert
Obesity.2025; 33(10): 1984. CrossRef - Association Between Heavy Metals Exposure and Elevated High-Sensitivity C-Reactive Protein: Mediating Role of Body Mass Index
Seong-Uk Baek, Jin-Ha Yoon
Biomolecules.2025; 15(11): 1491. CrossRef - Association between heavy metal exposure and biomarkers for non-alcoholic fatty liver disease in Korean adolescents
Dong-Wook Lee, Jongmin Oh, Yu Min Lee, Hyun-Joo Bae, Youn-Hee Lim
Heliyon.2024; 10(19): e37840. CrossRef
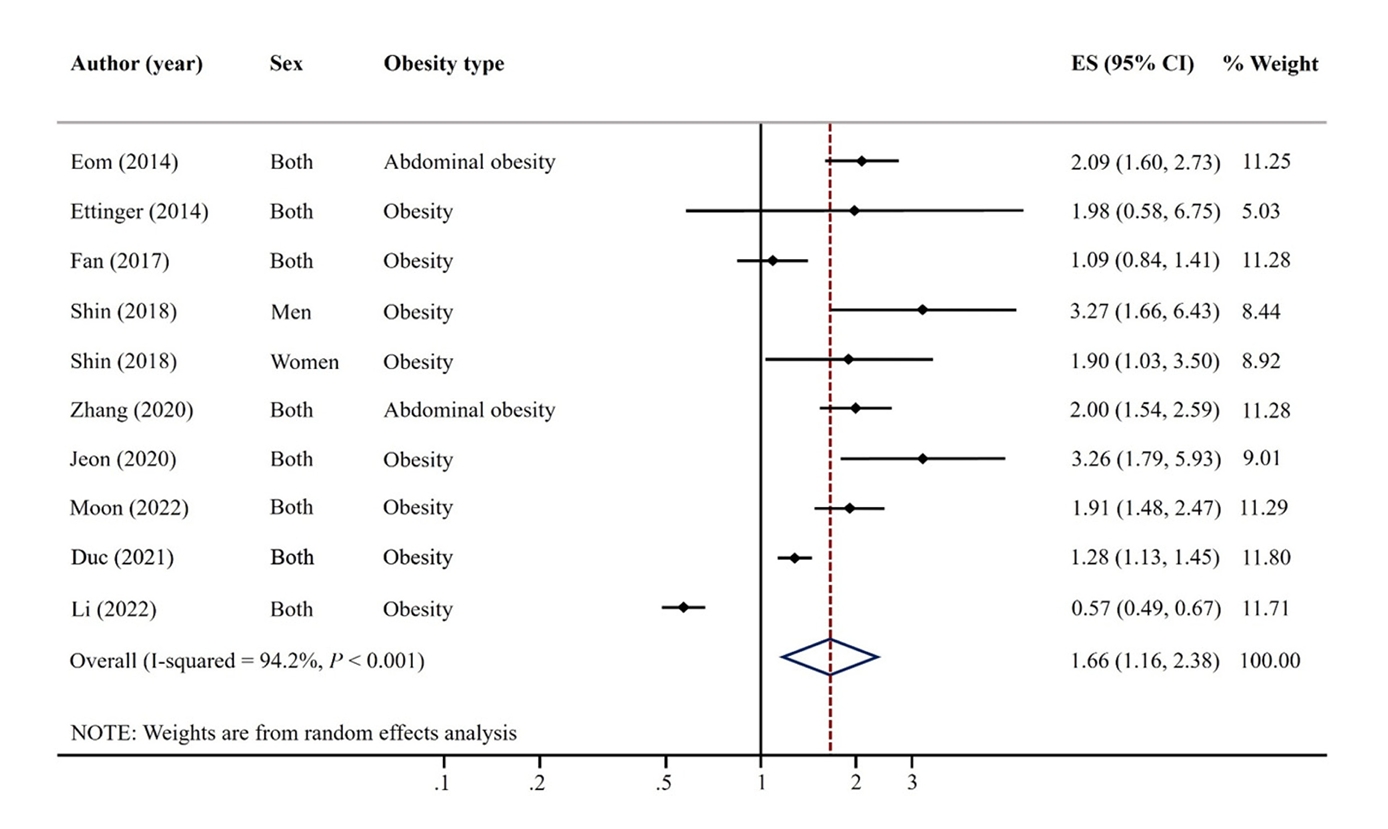
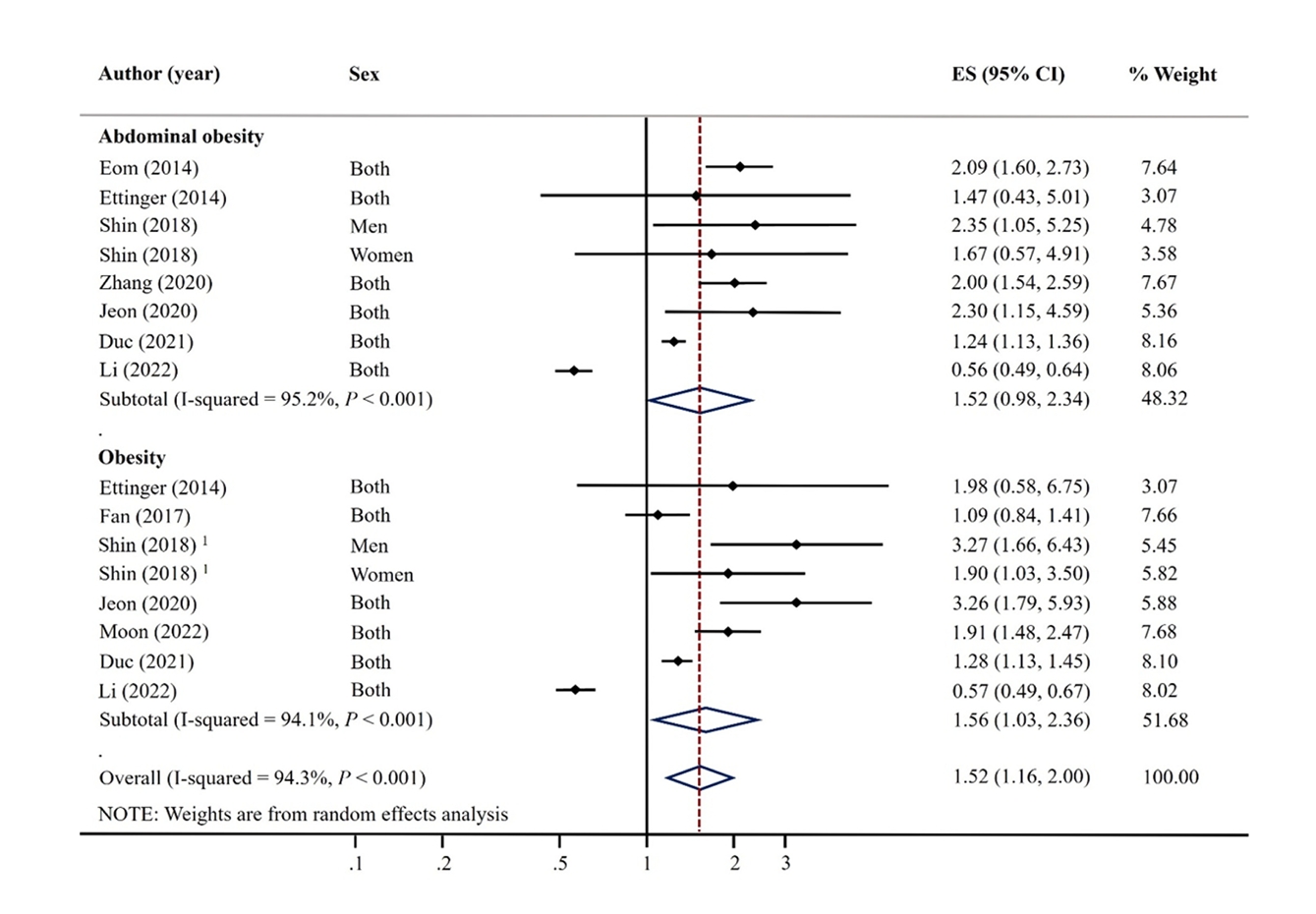
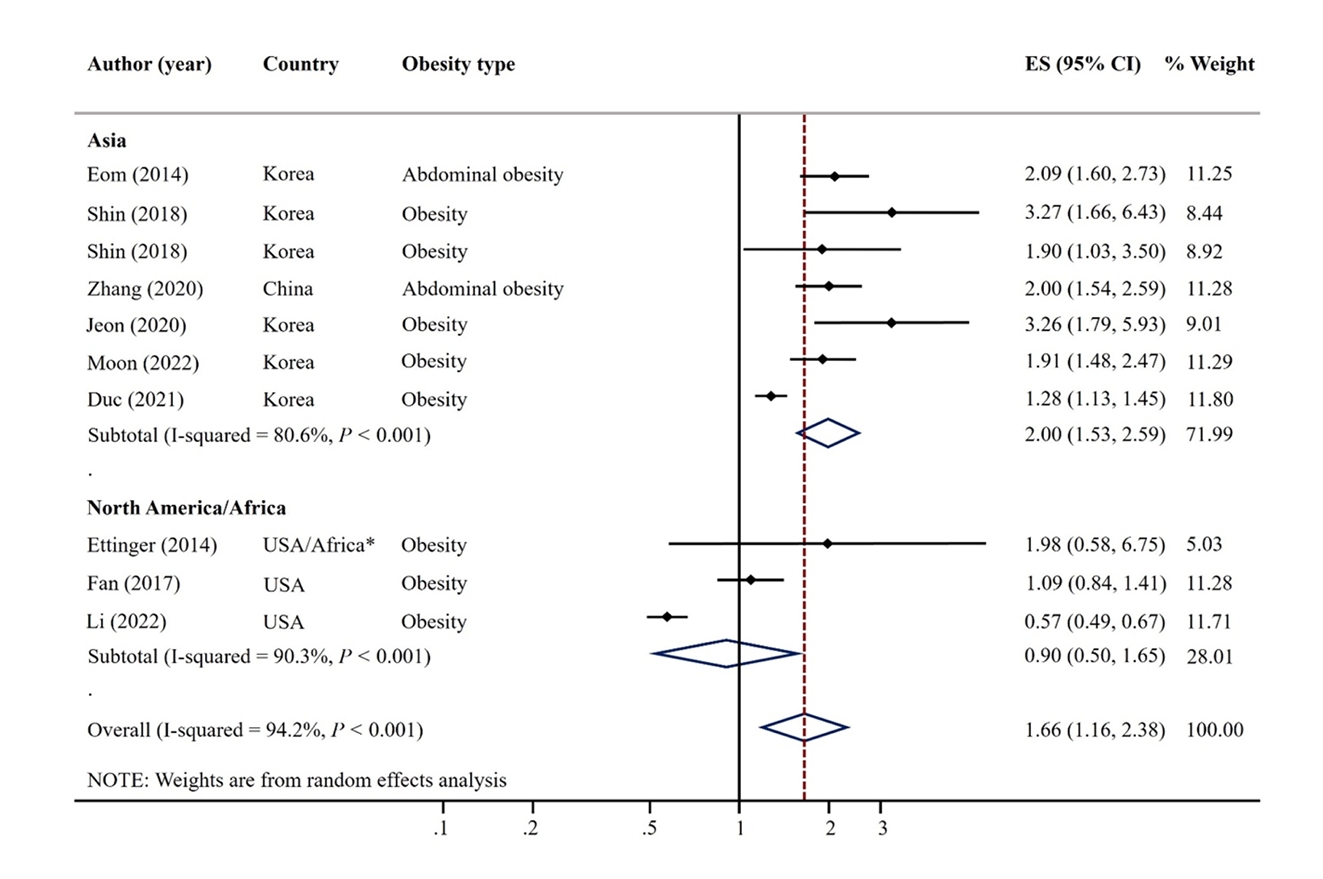
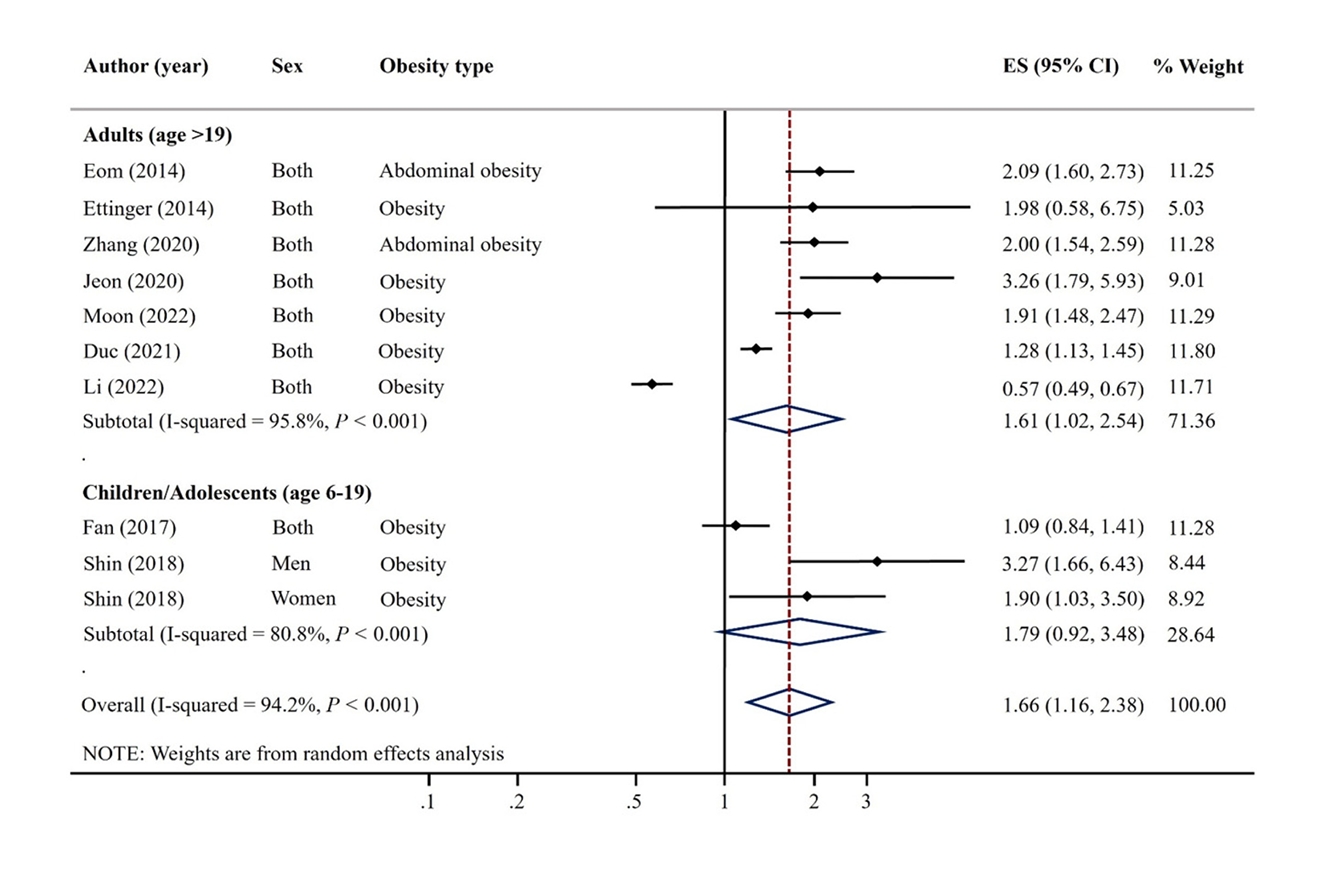
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Population | Children, adolescents, and adults |
| Intervention / Exposure | Mercury |
| Comparison | Highest vs. lowest categories of exposure |
| Outcomes | Obesity, abdominal obesity |
| Study design | Observational study designs (cross-sectional and case-control studies were available on this topic) |
| Author (year) | Country | Design | Sample size | Participant/Study name | Recruit period (year) | Sex | Mean or range of age (years) | Exposure | Outcome | Obesity definition | OR/PR (95% CI) | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eom (2014) [14] | Korea | Cross-sectional | 2,114 | Adults from metropolitan and rural districts | 2010-2011 | M | 45.5 | Blood mercury (μg/L) | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.09 (1.60, 2.72) | Sex, age, smoking, alcohol drinking, household income, residence area, seafood intake |
| W | ||||||||||||
| Ettinger (2014) [24] | Ghana, South Africa, Seychelles, Jamaica, USA | Cross-sectional | 150 | METS | 2010-2011 | M | 25-45 | Blood mercury (μg/L) | Obesity | BMI ≥ 25 kg/m2 | 1.98 (0.58, 6.74) | Sex, age, residence area, marital status, education level, paid employment, smoking, alcohol drinking, fish intake |
| W | Abdominal obesity | WC ≥ 94 cm for men, ≥ 80 cm for women | 1.47 (0.43, 5.00) | |||||||||
| Fan (2017) [26] | USA | Cross-sectional | 5,404 | NHANES | 2011-2014 | M | 6-19 | Blood mercury (μg/L) | Obesity | BMI percentile | 1.09 (0.84, 1.41) | Sex, age, race, poverty income ratio, TV, computer, and video games use in hours, BMI |
| W | ||||||||||||
| Shin (2018) [15] | Korea | Cross-sectional | 1,567 (M 793 W 774) | KNHANES | 2010-2013 | M | 15.0 | Blood mercury (μg/L) | Overweight/obesity | 1) BMI ≥ 85th percentile (aged < 19 years) | M 3.27 (1.66, 6.41) | Age, household income, energy intake, fulfillment of moderateto-vigorous physical activity |
| 2) BMI ≥ 23 kg/m2 (aged 19 years) | W 1.90 (1.03, 3.49) | |||||||||||
| W | Abdominal obesity | WHR ≥ 0.5 | M 2.35 (1.05, 5.24) | |||||||||
| W 1.67 (0.57, 4.93) | ||||||||||||
| Zhang (2020) [19] | China | Case-control | 4,134 | Beijing Population Health Cohort study | 2017 | M | 50-75 | Blood mercury (μg/L) | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.00 (1.55, 2.60) | Education level, smoking, alcohol drinking, BMI, physical activity, family history of disease |
| W | ||||||||||||
| Jeon (2021) [16] | Korea | Cross-sectional | 495 | SELEN | 2012-2013 | M | 40-69 | Toenail mercury (μg/g) | Obesity | BMI ≥ 25 kg/m2 | 3.26 (1.79, 5.93) | Age, sex, education level, residential area, monthly household income, smoking status, alcohol consumption, physical activity, total energy intake, use of dietary supplements |
| W | Abdominal obesity | WC ≥ 90 cm for men, ≥ 80 cm for women | 2.30 (1.15, 4.59) | |||||||||
| Moon (2022) [17] | Korea | Cross-sectional | 3,787 | KoNHES | 2015-2017 | M | ≥ 19 | Blood mercury (μg/L) | Obesity | BMI ≥ 25 kg/m2 | 1.91 (1.48, 2.47) | Age, sex, smoking, alcohol drinking, exercise, education levels |
| Duc (2021) [18] | Korea | Cross-sectional | 6,434 | KNHANES | 2009-2017 | M | ≥ 50 | Blood mercury (μg/L) | Obesity | BMI ≥ 27.5 kg/m2 | 1.28 (1.13, 1.45) | Comorbidities, sex, age, energy intake, occupation, family history of hyperlipidemia, family history of CVD, physical activity, drinking status, residential areas, smoking, ln2 cotinine, educational level, and monthly household incomes |
| W | Abdominal obesity | WC ≥ 90 cm for men or ≥ 80 cm for women | 1.24 (1.13, 1.36) | |||||||||
| Li (2022) [25] | USA | Cross-sectional | 15,959 | NHANES | 2007-2018 | M | ≥ 20 | Blood mercury (μg/L) | Obesity | BMI ≥ 30 kg/m2 | 0.57 (0.49, 0.67) | Sex, age, race, income, education, marriage, smoking, drinking status, physical activity, diabetes, cardiovascular disease |
| W | Abdominal obesity | WC ≥ 102 cm for men or ≥ 88 cm for women | 0.56 (0.49, 0.65) |
PICOS, population, intervention/exposure, comparison, outcomes, study design
OR, odds ratio; PR, prevalence ratio; CI, confidence interval; M, men; W, women; KNHANES, Korea National Health and Nutrition Examination Survey; KoNHES, Korean National Environmental Health Survey; NHANES, National Health and Nutrition Examination Survey; METS, Modeling the Epidemiologic Transition Study; SELEN, Trace Element Study of Korean Adults in the Yeungnam area

 KSCN
KSCN

 Cite
Cite