Search
- Page Path
- HOME > Search
Research Article
- [English]
- Effects of a multi-component program based on partially hydrolyzed guar gum (Sunfiber®) on glycemic control in South Korea: a single-arm, pre-post comparison pilot clinical trial
- Hyoung Su Park, A-Hyun Jeong, Hyejung Hong, Hana Jang, Hye-Jin Kim
- Korean J Community Nutr 2025;30(1):40-52. Published online February 28, 2025
- DOI: https://doi.org/10.5720/kjcn.2024.00276
- Correction in: Korean J Community Nutr 2025;30(2):173
-
 Abstract
Abstract
 PDF
PDF - Objectives
The aim of this study was to assess the impact of a multi-component program, including partially hydrolyzed guar gum (PHGG, Sunfiber®) supplementation, on glycemic control, gut health, and nutritional status to support diabetes prevention and management among Korean adults.
Methods
A single-arm trial was conducted with 29 adults (aged 20-55 years) with fasting plasma glucose (FPG) ≥ 100 mg/dL. Over a six-week period, participants engaged in a multi-component program that incorporated the supplementation of PHGG (Sunfiber®, 12.5 g/day), weekly nutritional coaching, and the use of continuous glucose monitoring devices. The program’s effectiveness was evaluated by measuring FPG and glycated hemoglobin (HbA1c) levels through blood tests conducted before and after the intervention. Improvements in gut health were gauged using the Korean Gut Quotient Measurement Scales, while enhancements in nutritional status were assessed using the Nutrition Quotient (NQ) and surveys that evaluated improvements in gut health and nutritional status.
Results
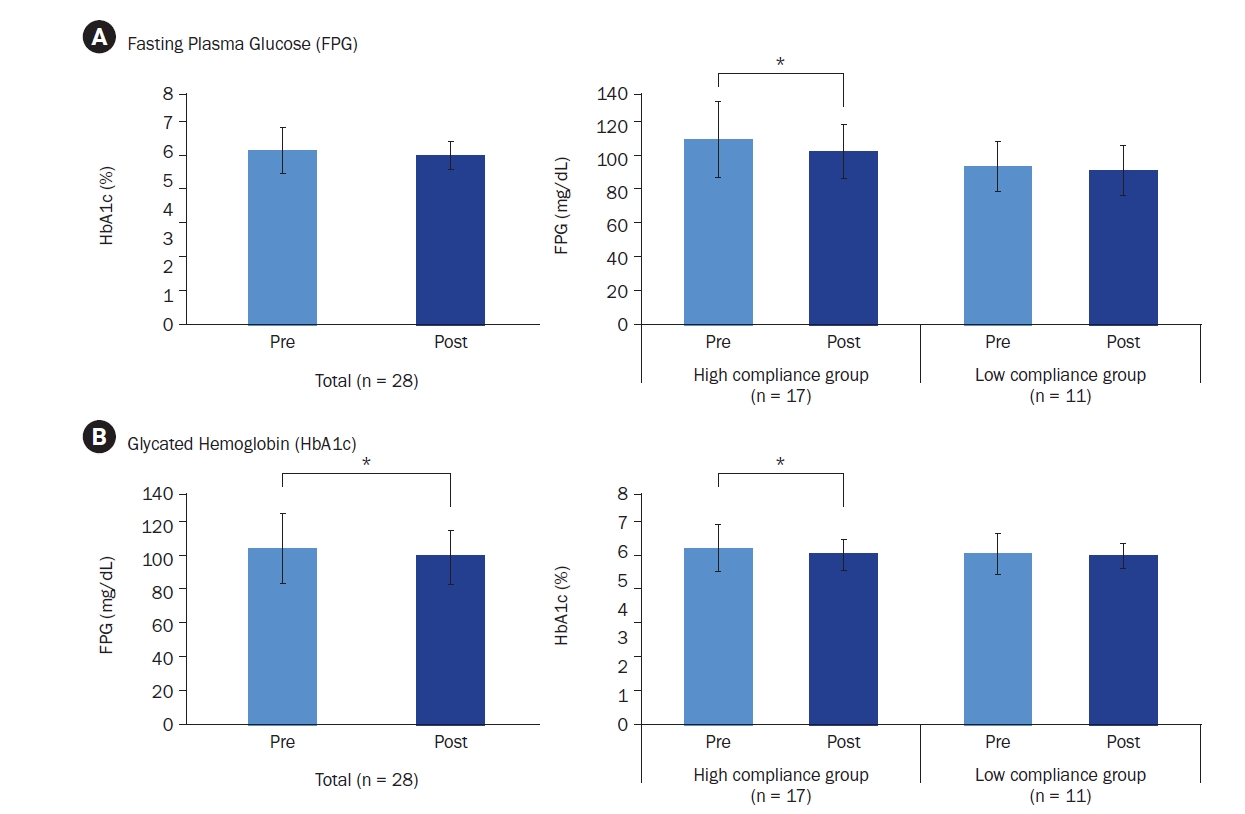
Participants’ average age was 43.89 years, with approximately 80% being male. Most participants (about 75%) were classified as overweight or obese. After six-weeks, 17 participants who adhered closely to the program (meeting certification criteria) exhibited significant reductions in key blood glucose markers. FPG levels decreased from 113.06 ± 23.16 mg/dL to 106.24 ± 16.33 mg/dL (P < 0.05), and HbA1c levels decreased from 6.08% ± 0.81% to 5.87% ± 0.53% (P < 0.05). The NQ evaluation revealed significant increases in comprehensive nutrition scores, and in the balance and practice domain scores for all participants (P < 0.05). Furthermore, in the gut health survey, approximately 82.1% of all participants reported experiencing positive changes.
Conclusion
Among adults with elevated FPG levels, a multi-component intervention program that included PHGG (Sunfiber®) supplementation, structured dietary management, and the use of health-monitoring devices showed significant benefits in improving glycemic control, overall nutritional status, and gut health. Trial Registration: Clinical Research Information Service Identifier: KCT0010049. -
Citations
Citations to this article as recorded by- Comparative analysis of dietary and lifestyle habits according to the prediabetic status in young adults
Joungyoon Seo, SeongHee Shin, Yuri Kim, Yoo Kyoung Park
Journal of Nutrition and Health.2025; 58(5): 468. CrossRef - Partially Hydrolyzed Guar Gum Combined with a Low-Fat Diet Ameliorates Type 2 Diabetes Mellitus via Modulating Gut Microbiota and Fecal Metabolites
Zhiqiang Cao, Hongxia Li, Quantao Cai, Li Chen, Liangzhong Liu, Yuhan Tang, Zhe Zhu, Ping Yao
Nutrients.2025; 17(23): 3746. CrossRef
- Comparative analysis of dietary and lifestyle habits according to the prediabetic status in young adults
- 7,584 View
- 45 Download
- 2 Crossref


 KSCN
KSCN

 First
First Prev
Prev