Abstract
-
Objectives
- The aim of this study was to assess the impact of a multi-component program, including partially hydrolyzed guar gum (PHGG, Sunfiber®) supplementation, on glycemic control, gut health, and nutritional status to support diabetes prevention and management among Korean adults.
-
Methods
- A single-arm trial was conducted with 29 adults (aged 20-55 years) with fasting plasma glucose (FPG) ≥ 100 mg/dL. Over a six-week period, participants engaged in a multi-component program that incorporated the supplementation of PHGG (Sunfiber®, 12.5 g/day), weekly nutritional coaching, and the use of continuous glucose monitoring devices. The program’s effectiveness was evaluated by measuring FPG and glycated hemoglobin (HbA1c) levels through blood tests conducted before and after the intervention. Improvements in gut health were gauged using the Korean Gut Quotient Measurement Scales, while enhancements in nutritional status were assessed using the Nutrition Quotient (NQ) and surveys that evaluated improvements in gut health and nutritional status.
-
Results
- Participants’ average age was 43.89 years, with approximately 80% being male. Most participants (about 75%) were classified as overweight or obese. After six-weeks, 17 participants who adhered closely to the program (meeting certification criteria) exhibited significant reductions in key blood glucose markers. FPG levels decreased from 113.06 ± 23.16 mg/dL to 106.24 ± 16.33 mg/dL (P < 0.05), and HbA1c levels decreased from 6.08% ± 0.81% to 5.87% ± 0.53% (P < 0.05). The NQ evaluation revealed significant increases in comprehensive nutrition scores, and in the balance and practice domain scores for all participants (P < 0.05). Furthermore, in the gut health survey, approximately 82.1% of all participants reported experiencing positive changes.
-
Conclusion
- Among adults with elevated FPG levels, a multi-component intervention program that included PHGG (Sunfiber®) supplementation, structured dietary management, and the use of health-monitoring devices showed significant benefits in improving glycemic control, overall nutritional status, and gut health.
-
Trial Registration
-
Clinical Research Information Service Identifier: KCT0010049.
-
Keywords: multi-component program; partially hydrolyzed guar gum; glycemic control; nutritional status; diabetes mellitus, type 2
INTRODUCTION
Diabetes mellitus, recognized by the World Health Organization (WHO) as one of the four major non-communicable diseases, has seen a steady rise in both incidence and patient numbers in recent decades [1]. As of 2017, diabetes was responsible for approximately 4 million deaths, and by 2020, it was estimated that 460 million people worldwide were affected. This figure is projected to increase to 629 million by 2045 [2].
As per the Diabetes Fact Sheet in Korea 2024, published recently by the Korean Diabetes Association, as of 2022, one in seven adults (14.8%) aged 30 and above had diabetes mellitus. The prevalence increased to three in ten (28.0%) among those aged 65 and above [2]. This is nearly double the 3.2 million diabetes mellitus patients reported in 2010. Moreover, the prevalence of prediabetes suggests that four in ten adults (41.1%) aged 30 and above, and nearly half (47.7%) of the elderly population aged 65 and above, were in a prediabetic state. These statistics emphasize a higher prevalence of prediabetes among the elderly and highlight the growing importance of diabetes prevention and management in an aging society [2-4].
Dietary fiber intake plays a vital role in managing diabetes due to its effects on gut health and glycemic control [5, 6]. Research has shown that dietary fiber positively impacts metabolic health through several mechanisms, including promoting beneficial gut bacteria, improving bowel movements, suppressing postprandial blood glucose elevation, and enhancing blood cholesterol profiles [7, 8]. Specifically, in cases of type 2 diabetes, where poor dietary habits are a primary cause, dietary interventions, such as dietary fiber supplementation, are considered more effective core management strategies than exercise interventions [9].
Gut health is essential for blood glucose regulation and diabetes management. An imbalance in the gut microbiota can trigger inflammatory responses, which are significant factors in increasing insulin resistance and reducing glycemic control [10]. Dietary fiber intake is known to encourage the growth of beneficial gut bacteria, thereby enhancing the intestinal environment and contributing to the suppression of blood glucose and improved insulin sensitivity [5, 11]. This approach, centered on gut health, is believed to have positive effects on glycemic control and overall metabolic health.
Comprehensive lifestyle modifications are essential for fundamental diabetes prevention and management. These modifications include changes in dietary habits, physical activity, weight management, and stress control [12-14]. Regarding physical activity, a minimum of 150 minutes of moderate-intensity exercise per week is recommended. Research suggests that programs aiming for a 5% weight reduction over six months, combined with dietary control, are effective [13]. Modifications in dietary habits require caloric restriction, appropriate nutrient distribution, and ongoing nutritional management, supported by follow-up monitoring and telephone consultations [14]. Moreover, combining dietary intervention and exercise is a critical approach for preventing type 2 diabetes and reducing complications [15, 16].
Recent research has underscored the necessity and effectiveness of multi-component programs for diabetes prevention and management. These programs incorporate diet, exercise, physical activity, blood glucose monitoring, medication, medical examinations, and smoking cessation [17-19]. Studies have also demonstrated the effectiveness of IT-based monitoring, which combines medical and information technology convergence services with direct counseling and education to improve lifestyle habits [20]. These multi-component programs suggest that diabetes management can be enhanced through multifaceted approaches, including nutritional coaching and IT-based monitoring, beyond mere lifestyle modifications [21].
Therefore, this study aims to assess whether a multi-component program that combines dietary fiber supplementation, weekly nutritional coaching, and continuous glucose monitoring (CGM) can improve glycemic control, gut health, and nutritional status. The study focuses on adults aged 20 and above with fasting plasma glucose (FPG) levels of 100 mg/dL or higher who want to improve their blood glucose control.
METHODS
Ethics statement
All participants provided written informed consent for the study. The study procedures and protocols were approved by the Institutional Review Board (IRB) of the Public Bioethics Committee, recognized by the Minister of Health and Welfare (IRB No.: P01-202406-01-007), and the Clinical Research Information Service (approval number: KCT0010049).
1. Study design
This study was a single-arm, pre-post comparison pilot clinical trial, reported in accordance with the CONSORT (Consolidated Standards of Reporting Trials) 2010 extension guidelines for pilot and feasibility trials.
2. Study participants and recruitment
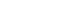
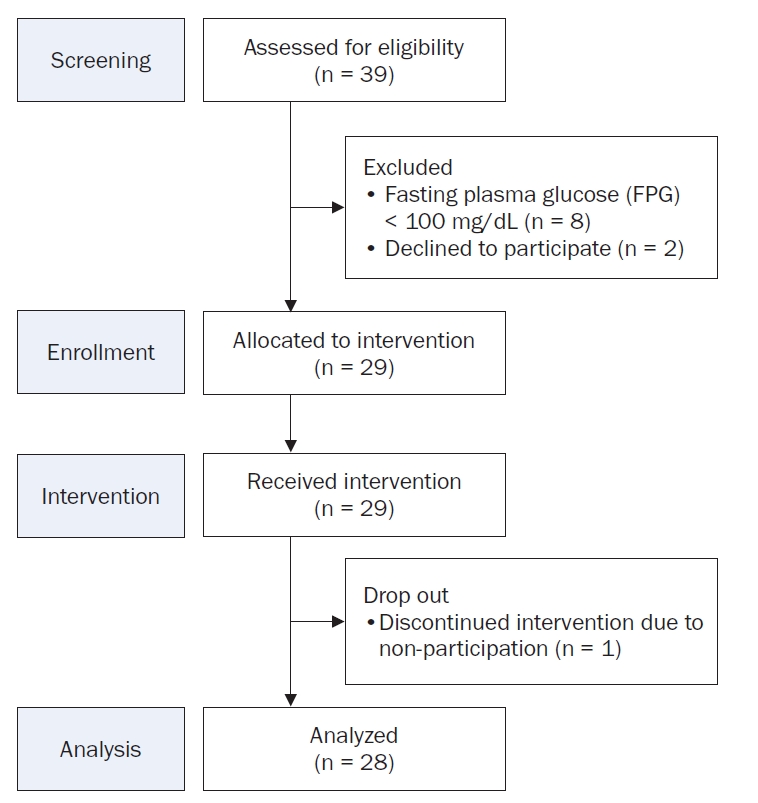
The study was conducted among employees of a single domestic corporation, targeting adults aged 20 to 55 who were interested in improving glycemic control. Participant recruitment was done through internal postings and emails, which provided information about the study’s objectives, selection criteria, and schedule, followed by voluntary application submissions. The recruitment period was from June 3 to June 14, 2024. Of the 39 individuals recruited, eight were excluded due to FPG levels below 100 mg/dL, and two withdrew consent. One of the 29 participants who entered the program dropped out, resulting in a final analysis sample of 28 participants (Fig. 1).
The inclusion criteria were as follows: (1) adults aged 20–55 years; (2) individuals with FPG levels ≥ 100 mg/dL, which corresponds to impaired fasting glucose (IFG), an indicator of the borderline status between normal glycemic levels and diabetes mellitus [22]; and (3) individuals who provided written informed consent for study participation.
The FPG criterion of ≥ 100 mg/dL was based on the definition of IFG presented in the 2023 Clinical Practice Guidelines for Diabetes Mellitus [22]. Using this criterion, the study aimed to evaluate the effectiveness of dietary fiber supplementation in early-risk groups classified as prediabetic.
Prospective participants submitted written consent forms to the researchers, who reviewed their health examination results from medical institutions within the past 12 months to verify compliance with the FPG criteria. Individuals with hypersensitivity to partially hydrolyzed guar gum (PHGG), a component of the dietary fiber supplement, or those with severe food allergy reactions, were excluded.
3. Intervention methods
The intervention program lasted six weeks and included dietary fiber supplementation, weekly nutritional coaching, and CGM. For dietary fiber supplementation, participants consumed PHGG, a type of soluble dietary fiber. Participants consumed 12.5 g of PHGG supplementation (Selex Sunfiber Guar gum Prebiotics [Sunfiber®], Maeil Health Nutrition Co., Ltd.) daily before meals. Compliance was monitored through a mobile application, where participants uploaded photos of their supplement intake and meal records at least twice daily. Researchers reviewed these records and provided personalized dietary feedback via a messaging platform.
Participants used the FreeStyle Libre (Abbott Diabetes Care Limited) CGM system to monitor real-time glucose fluctuations. Weekly nutritional coaching sessions offered individualized dietary guidance based on participants’ glycemic trends and meal records. This multi-component approach aimed to enhance self-awareness, optimize glycemic control, and foster sustainable dietary habits.
During the intervention period, study participants consumed a PHGG supplement (12.5 g once daily before meals). The supplement consisted entirely of PHGG (Sunfiber®), a standardized functional ingredient approved by the Food and Drug Administration for its potential benefits, including promotion of beneficial intestinal bacteria growth, improvement of bowel movements, suppression of postprandial blood glucose elevation, and improvement of blood cholesterol levels [23]. To enhance adherence and compliance, participants were required to upload daily photographs of their supplement consumption and dietary intake records (at least twice daily) via a mobile application (Selex, Maeil Health Nutrition Co., Ltd.) (Fig. 2). Researchers regularly reviewed these records and provided individualized feedback through the KakaoTalk open chat channels (Kakao Corp.), including weekly dietary consultations and personalized nutritional coaching to help participants maintain their planned intake goals. The nutritional coaching involved an evaluation of participants’ dietary records, focusing on nutrient intake, caloric content, and dietary balance. This assessment facilitated the identification of nutritional deficiencies or excesses and the development of specific dietary modification strategies. Furthermore, personalized advice and motivation were provided to ensure consistent intake patterns throughout the study period, supporting improved nutritional status and the formation of healthy lifestyle habits.
Participants were equipped with CGM devices (FreeStyle Libre) to track real-time fasting and postprandial glucose variations. This self-monitoring system increased awareness of glycemic control and promoted autonomous management. Researchers used this data to indirectly evaluate the effectiveness of the intervention on glycemic control.
4. Outcome measures
The study assessed blood parameters (FPG, glycated hemoglobin [HbA1c]), nutritional indices through questionnaires, gastrointestinal health, and program satisfaction, with evaluations conducted pre- and post-intervention. Initially, baseline characteristics, including sex, age, height, weight, and medication status, were collected through preliminary questionnaires. Blood parameters were measured at designated medical facilities. Participants underwent standardized testing after 8–10 hours of fasting to measure FPG and HbA1c levels.
Nutritional status was assessed using the Korean Nutrition Society’s nutrition quotient (NQ) questionnaire [24]. This questionnaire comprises 20 items that evaluate dietary habits and nutritional status comprehensively. Results were analyzed across three primary domains: balance, moderation, and practice, to determine the program's impact in each area.
Gastrointestinal health was evaluated using the Korean Gut Quotient Measurement Scale, which consists of five items measuring defecation frequency, timing, volume, and stool characteristics [25]. A post-intervention question was added to assess subjective changes in gastrointestinal health. The Cronbach’s alpha values for the NQ and Gut Quotient questionnaires were 0.680 and 0.437, respectively.
Finally, a satisfaction assessment was conducted post-intervention to evaluate participants' subjective perceptions of the program’s effectiveness and components. The five-item questionnaire analyzed glycemic control, gastrointestinal health improvements, and the utility of nutritional coaching and CGM.
5. Statistical analysis
Program effectiveness was evaluated through pre- and post-intervention measurements of FPG, HbA1c, nutritional indices, and gastrointestinal health questionnaires. Statistical analyses were performed using SPSS® statistical software (version 22; IBM Co.). Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as means and standard deviations. Participants were stratified into “high compliance” (≥ 70% dietary record participation and ≥ 80% supplement consumption verification) and “low compliance” groups, with additional analyses stratified by age (above/below 40 years) and sex.
Pre- and post-intervention differences for continuous variables were analyzed using paired t-tests or Wilcoxon’s signed-rank tests following normality testing. Effect sizes were calculated using Cohen’s d for paired t-tests and R-values for Wilcoxon’s signed-rank tests. Changes in categorical variables were analyzed using McNemar’s test following normality testing. All statistical tests were two-tailed, with significance set at P < 0.05. Statistically significant results were considered clinically meaningful.
RESULTS
1. Subject characteristics
The study included 28 participants with a mean age of 43.89 ± 6.80 years and a mean body mass index (BMI) of 26.64 ± 4.59 (data not shown in tables). The sample comprised 23 males (82.1%) and five females (17.9%), with the following age distribution: 20–29 (n = 1, 3.6%), 30–39 (n = 5, 17.9%), 40–49 (n = 16, 57.1%), and 50–55 years (n = 6, 21.4%), indicating a predominance of participants aged 40 and above (Table 1).
BMI was calculated using participants' height and weight and classified according to the obesity criteria from the WHO for the Asia-Pacific region and the Korean Society for the Study of Obesity. The distribution showed 7 participants (25.0%) with normal weight (BMI 18.5–22.9 kg/m2), 4 (14.3%) overweight (BMI 23.0–24.9 kg/m2), and 17 (60.7%) obese (BMI ≥ 25.0 kg/m2). Overall, 75.0% of participants were classified as overweight or obese (BMI ≥ 23.0 kg/m2). Regarding diabetes status, six participants (21.4%) had diabetes mellitus, while 22 (78.6%) did not. Four participants (14.3%) reported using health functional foods for blood glucose control, whereas 24 (85.7%) reported no supplement use.
Medication use, assessed through multiple-response items, was reported by 13 participants (46.4%). These included antihypertensive agents (n = 8), lipid-lowering agents (n = 7), antidiabetic agents (n = 2), and singular cases of cholesterol-lowering agents, alopecia treatment agents, thyroid disorder medications, and benign prostatic hyperplasia medications.
2. Blood parameters (FPG and HbA1c)
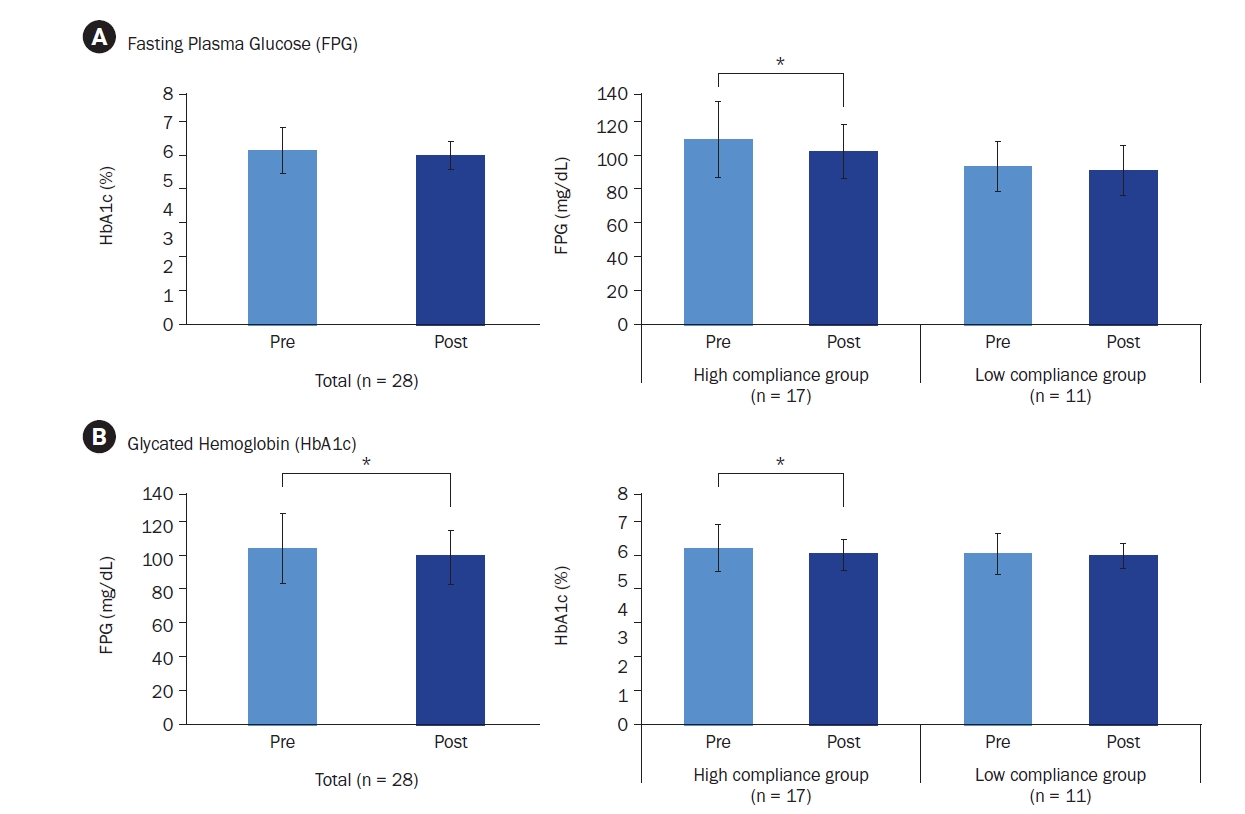
Pre- and post-intervention blood test results for FPG and HbA1c are presented in Fig. 3. and Fig. 4. Overall, FPG levels decreased from 106.68 ± 21.56 mg/dL to 101.54 ± 16.79 mg/dL, though this change was not statistically significant. Compliance-based analysis showed that the high compliance group (n = 17) exhibited a significant reduction in FPG from 113.06 ± 23.16 mg/dL to 106.24 ± 16.33 mg/dL (t = 2.162, Cohen’s d = –1.54, P = 0.039). The low compliance group (n = 11) showed a non-significant decrease from 96.82 ± 14.86 mg/dL to 94.27 ± 15.45 mg/dL (Fig. 3A).
HbA1c levels significantly improved in the total sample, decreasing from 6.01% ± 0.77% to 5.86% ± 0.49% (t = 2.114, Cohen’s d = –0.31, P = 0.044). The high compliance group showed a significant reduction from 6.08% ± 0.81% to 5.87% ± 0.53% (t = 2.483, Cohen’s d = –0.26, P = 0.012), while the low compliance group showed a non-significant decrease from 5.90% ± 0.72% to 5.84% ± 0.45% (Fig. 3B).
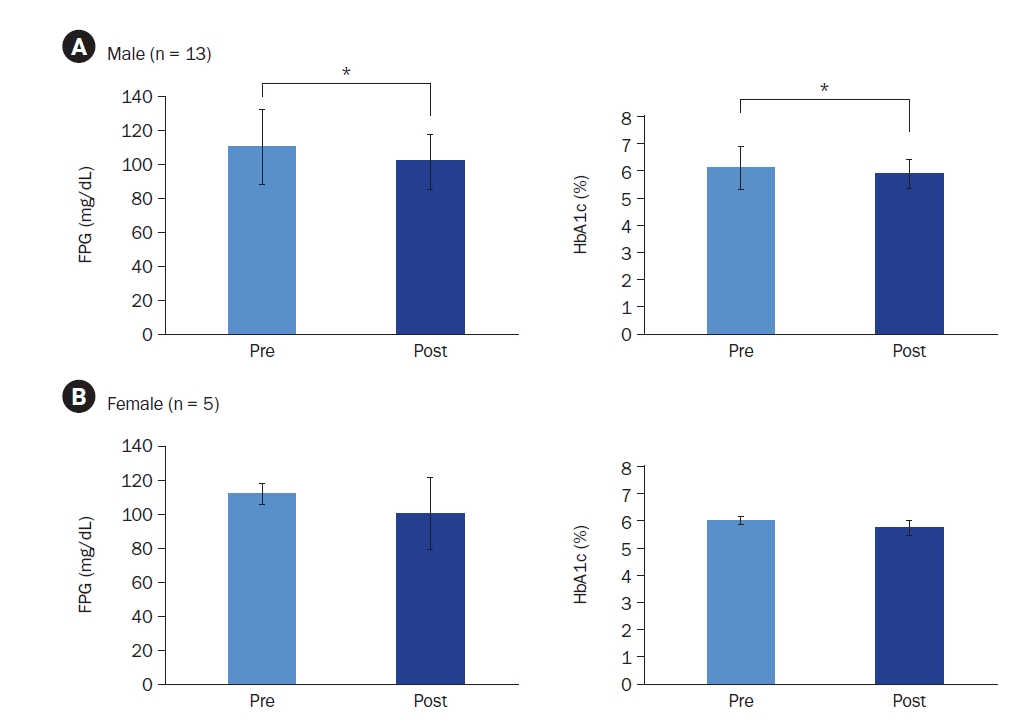
Additional stratified analyses were conducted, focusing on male participants (82%) and participants aged ≥ 40 years (78%) due to their high representation in the sample. Male participants, regardless of compliance level, demonstrated significant reductions in both FPG (110.35 ± 22.00 mg/dL to 101.74 ± 16.26 mg/dL; t = 2.597, Cohen’s d = –1.97, P = 0.014) and HbA1c (6.11% ± 0.81% to 5.90% ± 0.52%; t = 2.496, Cohen’s d = –0.41, P = 0.015) (Fig. 4A). Female participants showed no significant changes. Similarly, participants aged ≥ 40 years demonstrated significant reductions in FPG (110.68 ± 22.23 mg/dL to 104.27 ± 17.56 mg/dL; z = –1.979, r = –0.42, P = 0.048) and HbA1c (6.11% ± 0.84% to 5.90% ± 0.54%; z = –2.191, r = –0.47, P = 0.028), whereas those < 40 years showed no significant changes.
3. Nutrition quotient assessment
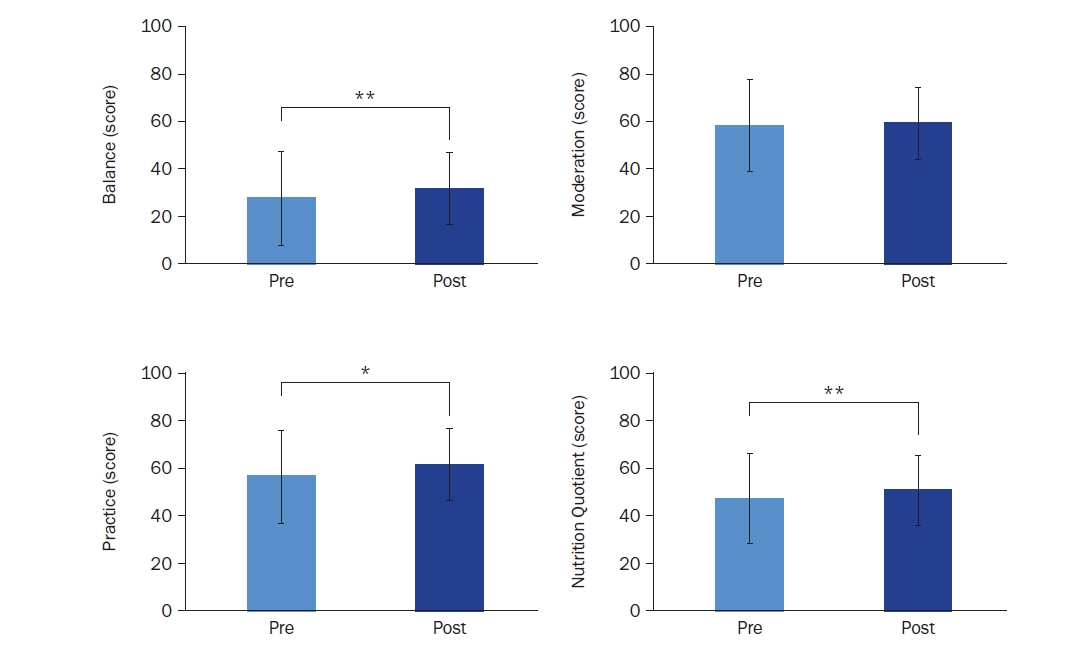
The overall NQ and subdomain scores (balance, moderation, and practice) are presented in Fig. 5. The total NQ score significantly improved from 47.80 ± 10.24 to 51.27 ± 10.65 (t = –2.585, Cohen’s d = –1.11, P = 0.004). Subdomain analysis revealed significant improvements in balance (27.33 ± 12.02 to 31.24 ± 13.32; t = –3.505, Cohen’s d = –1.08, P = 0.002) and practice (55.73 ± 13.89 to 60.78 ± 15.39; t = –2.585, Cohen’s d = –1.39, P = 0.015), while moderation showed a non-significant increase from 57.70 ± 13.09 to 58.61 ± 13.13.
4. Gastrointestinal health assessment
Pre- and post-intervention gastrointestinal health indicators are presented in Table 2. The number of participants reporting “no symptoms” increased from nine to 12, while those reporting “mild symptoms” increased from 11 to 14. Participants reporting “severe symptoms” decreased from eight to two, indicating overall improvement. Stool consistency assessments showed stable numbers for “very hard” and “hard, pellet-like” responses (n = 1 both pre- and post-intervention), while those reporting “smooth, sausage-like” increased from nine to 11, suggesting normalization of stool consistency. Analysis of defecation frequency showed a slight decrease in the number of participants with daily bowel movements, from 17 before the program to 14 after. In contrast, the number of participants with a bowel movement frequency of five to six times per week increased from five to nine. Defecation duration remained relatively stable for those completing within 5 minutes, with a reduction in those requiring 10–15 minutes or longer. Stool volume increased, with more participants reporting “1–2 cups” (16 to 21) and fewer reporting “less than 1 cup” (4 to 1). However, these changes were not statistically significant.
Post-intervention satisfaction survey results (Table 3) indicated that 82.1% of participants (n = 23) reported positive changes in gastrointestinal health, with three reporting “greatly improved,” six “improved,” and 14 “slightly improved.” Three participants reported “no change,” two reported “slightly worse,” and none reported “worse” or “much worse.”
5. Satisfaction survey
Table 4 presents the program satisfaction survey results. Of the participants, 13 reported being “very satisfied,” 12 “satisfied,” and three “neutral.” No participants reported being “dissatisfied” or “very dissatisfied.” The 83.3% positive response rate and absence of negative responses indicate high overall program satisfaction.
Satisfaction with specific program components was evaluated as follows (Table 5). Regarding blood glucose management through PHGG supplementation, four participants responded “strongly agree,” 15 “agree,” seven “neutral,” and two “disagree.” For nutritional coaching's impact on blood glucose management, nine responded “strongly agree,” 15 “agree,” and four “neutral.” All participants positively evaluated the utility of CGM for self-management, with 15 responding “strongly agree” and 13 “agree.” Regarding PHGG supplementation’s contribution to gastrointestinal health improvement, nine responded “strongly agree,” 13 “agree,” and six “neutral.”
DISCUSSION
This pilot study investigated the effects of a six-week multi-component program combining PHGG supplementation, nutritional coaching, and CGM on glycemic control among adults aged 20–55 years with FPG levels ≥ 100 mg/dL. Of the 29 initially enrolled participants who met the selection criteria, 28 completed the study, with one withdrawal. The participant cohort was predominantly male (≤ 80%) and had a mean age of 43.89 years, with over 75% aged 40–50. Approximately 60% were classified as overweight or obese based on their BMI.
Post-intervention assessment revealed significant reductions in both FPG and HbA1c among high compliance participants, with significant HbA1c improvements observed across the entire cohort. Despite the relatively brief six-week intervention period, these improvements likely resulted from the synergistic effects of pre-meal PHGG supplementation, twice-daily meal documentation, CGM, and weekly nutritional coaching, which collectively enhanced glycemic control awareness and self-management.
Previous research has demonstrated strong correlations between dietary fiber intake and glycemic control [26-28], with inverse associations between fiber intake and cardiovascular disease and stroke risk factors [29-31]. The Korea Disease Control and Prevention Agency recommends adequate dietary fiber intake, citing its role in moderating nutrient absorption rates and reducing cholesterol levels, thereby supporting glycemic control and cardiovascular disease prevention [32]. However, since the 1970s, the westernization of Korean dietary patterns has led to increased caloric and fat intake while reducing plant-based food consumption, resulting in fiber deficiency and rising chronic disease rates, including diabetes mellitus [33, 34]. Dietary supplements may, therefore, be beneficial when dietary fiber intake is insufficient. Among approximately six dietary fiber sources designated by the Food and Drug Administration, PHGG is registered as a functional ingredient [23]. Vuorinen-Markkola et al. [35] reported that patients with diabetes receiving 20 g daily of PHGG for six weeks showed 19.5% and 7.2% reductions in FPG and HbA1c, respectively. Similar benefits were observed in healthy individuals consuming 30 g daily, with a 6.25% reduction in FPG and a 7.3% reduction in blood cholesterol [36].
The integration of CGM with daily dietary recording and weekly personalized nutritional coaching via mobile platforms provided real-time feedback and individualized support, enhancing dietary compliance and self-management capabilities. Healthcare devices enabling real-time data monitoring have positively impacted dietary adherence and physical activity [37]. Mobile-based nutritional coaching has gained prominence post-COVID-19, utilizing digital technologies for remote interaction, video education, virtual consultations, and messaging [16, 38].
Beyond glycemic control, the study evaluated overall nutritional status and gastrointestinal health improvements. PHGG supplementation aids not only glycemic regulation but also promotes intestinal motility, prevents constipation, and supports dietary compliance through enhanced satiety [26]. Survey results demonstrated significant improvements in overall NQ scores, particularly in the balance and practice domains. Gastrointestinal health showed general improvement across defecation frequency, duration, volume, and consistency.
These findings suggest that effective chronic disease prevention and glycemic control in prediabetic and diabetic individuals require comprehensive management strategies addressing multiple modifiable lifestyle factors rather than singular interventions. Such multi-component approaches demonstrate potential benefits not only for primary health concerns but also for related health outcomes.
Limitations
This study has several limitations. First, the single-group pre-post design may affect internal validity due to potential confounding variables. Second, participant recruitment from a single institution and the relatively small sample size may limit the generalizability of the findings. Nevertheless, despite these limitations, this study provides valuable preliminary evidence suggesting that a multi-component program combining consistent PHGG supplementation, nutritional coaching-based dietary management, and health device utilization (CGM) can benefit not only FPG and HbA1c management but also overall health status, including nutritional status and gastrointestinal health, among prediabetic and diabetic individuals.
Conclusion
Comprehensive and proactive management strategies incorporating PHGG supplementation, dietary management, and health device utilization may be more effective for diabetes prevention and management than single-intervention approaches. The results of this study provide foundational evidence supporting the potential applicability of such multi-component approaches in community-based chronic disease management. Building upon these findings, we recommend conducting larger-scale studies to verify the efficacy of PHGG-based multi-component programs for diabetes prevention and management, with the goal of establishing this approach as a viable strategy for community-based chronic disease prevention.
NOTES
-
CONFLICT OF INTEREST
This study was conducted using Selex Sunfiber Guar gum Prebiotics (Sunfiber®) from Maeil Health Nutrition Co., Ltd. However, there are no financial or other issues that might lead to a conflict of interest.
-
FUNDING
None.
-
DATA AVAILABILITY
Research data is available upon request to the corresponding author.
Fig. 1.Participant flowchart for the single-arm trial.
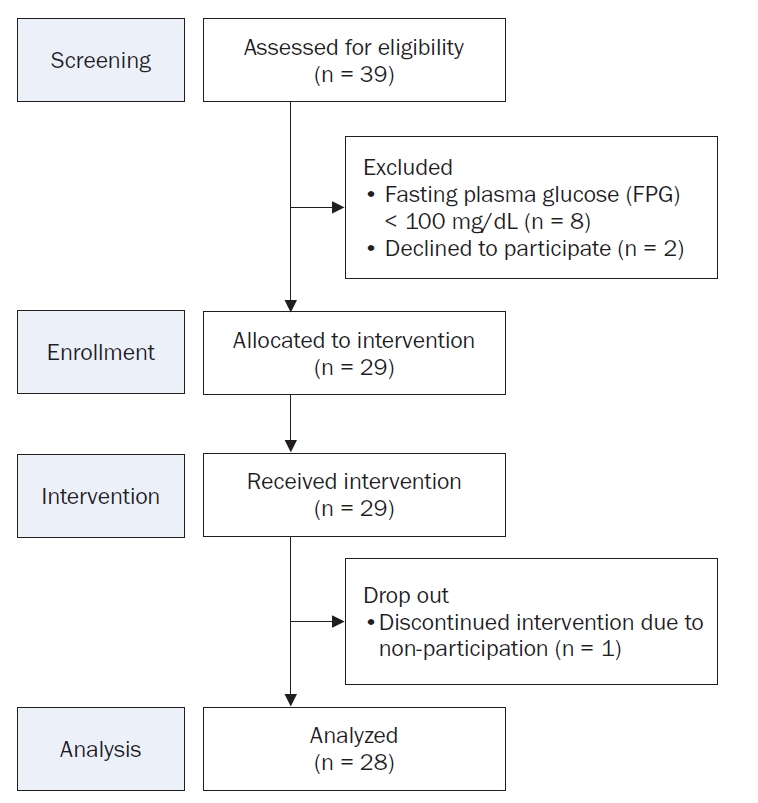
Fig. 2.Method for partially hydrolyzed guar gum (PHGG, Sunfiber®) supplementation and meal recording using the selex app.
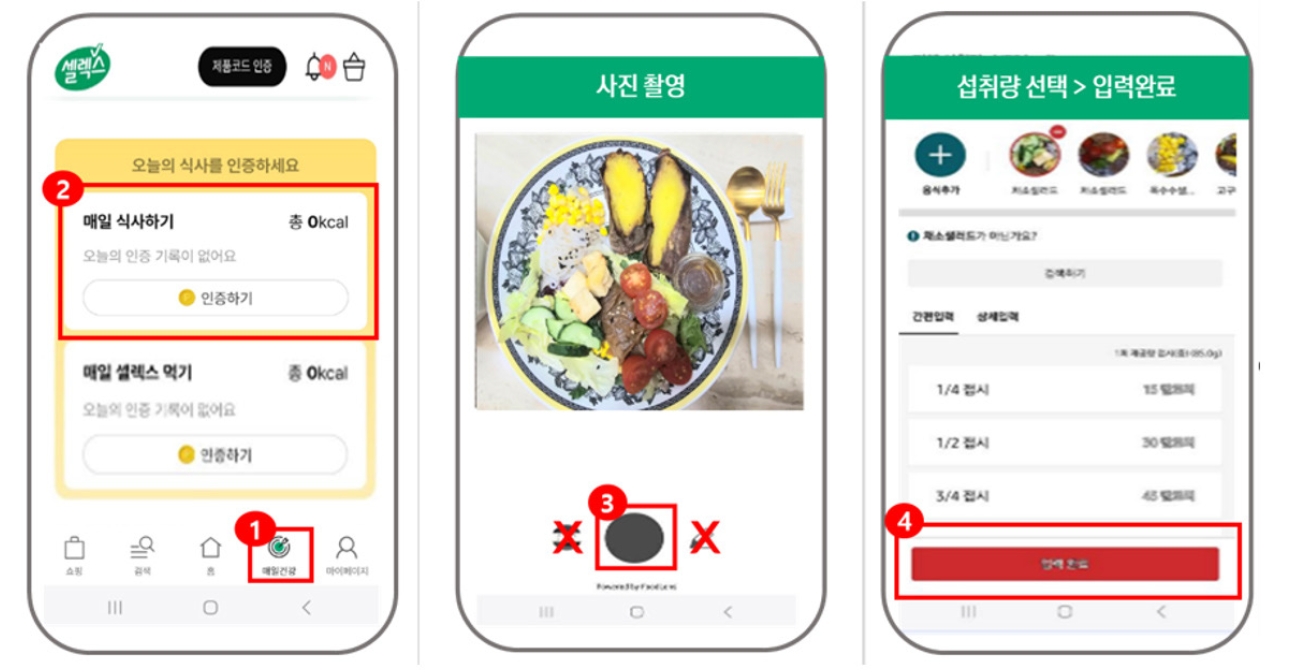
Fig. 3.
Changes in (A) fasting plasma glucose and (B) glycated hemoglobin levels in among all participants and by compliance status. Mean ± SD.
*Indicates a significant difference at P < 0.05. P-values were determined using a paired t-test or Wilcoxon’s signed-rank test, depending on normality.
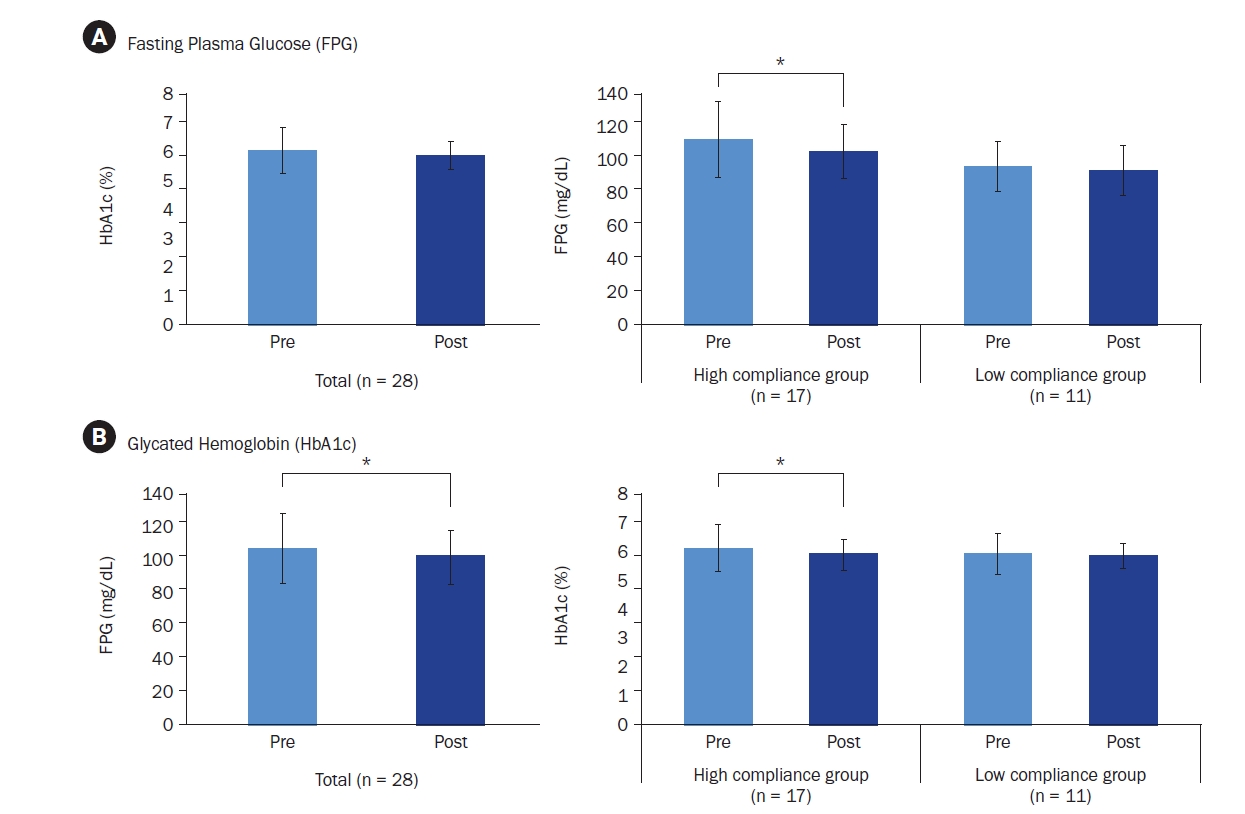
Fig. 4.
Changes in fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) levels in (A) male and (B) female participants. Mean ± SD.
*Indicates a significant difference at P < 0.05. P-values were determined using a paired t-test or Wilcoxon’s signed-rank test, depending on normality.
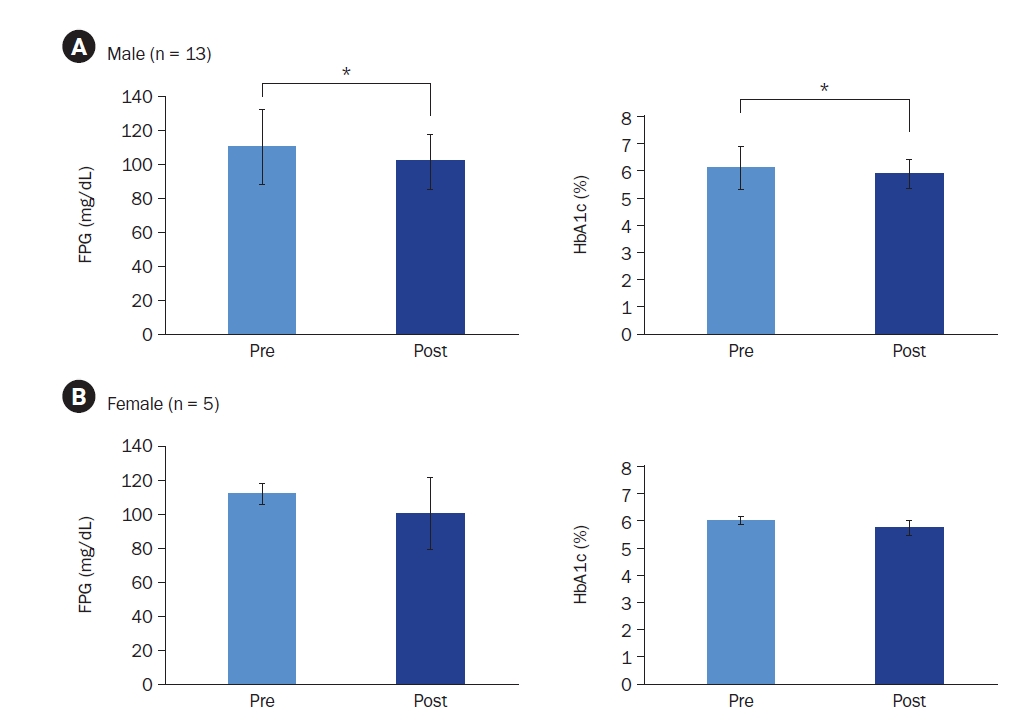
Fig. 5.
Changes in nutrition scores based on nutrition quotient (NQ) assessment: balance, moderation, practice, and nutrition quotient (n = 28). Mean ± SD.
*Indicates a significant difference at P < 0.05 and **indicates a significant difference at P < 0.01. P-values were determined using a paired t-test or Wilcoxon’s signed-rank test, depending on normality.
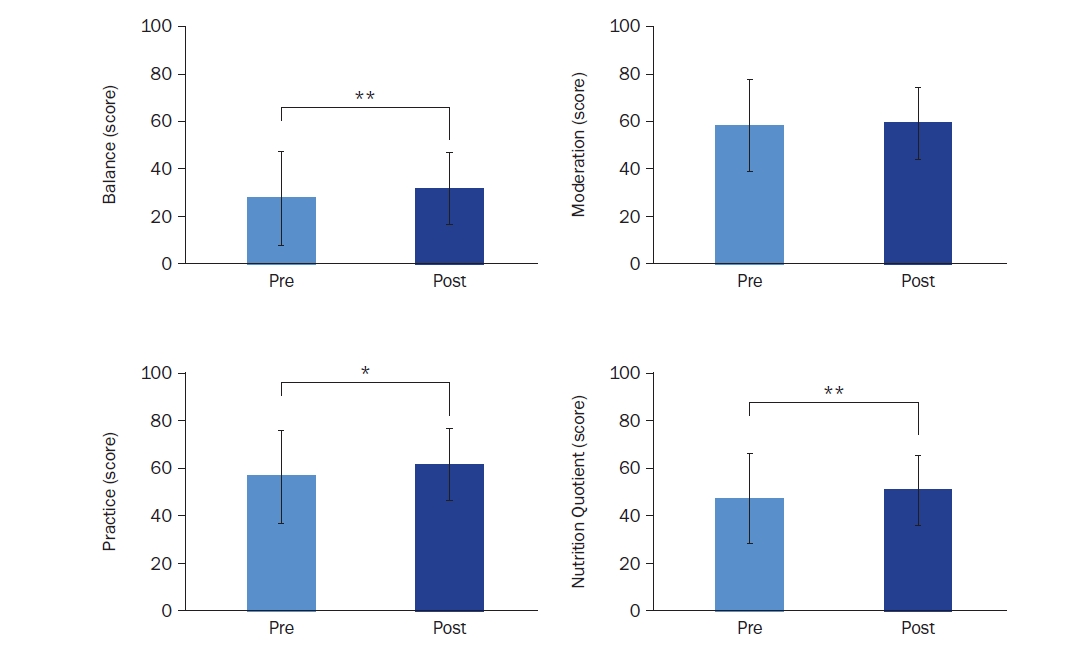
Table 1.Baseline characteristics of study participants
|
Variable |
Subject (n = 28) |
|
Sex |
|
|
Male |
23 (82.1) |
|
Female |
5 (17.9) |
|
Age group (year) |
|
|
20–29 |
1 (3.6) |
|
30–39 |
5 (17.9) |
|
40–49 |
16 (57.1) |
|
50–55 |
6 (21.4) |
|
Body mass index |
|
|
Underweight |
0 (0.0) |
|
Normal weight |
7 (25.0) |
|
Overweight |
4 (14.3) |
|
Obese |
17 (60.7) |
|
Diabetes status |
|
|
Diabetes present |
6 (21.4) |
|
Diabetes absent |
22 (78.6) |
|
Intake of health functional food for blood glucose control |
|
|
Yes |
4 (14.3) |
|
No |
24 (85.7) |
|
Medication type (multiple responses allowed) |
|
|
Antihypertensive agents |
8 (28.6) |
|
Lipid-lowering agents |
7 (25.0) |
|
Antidiabetic agents |
2 (7.1) |
|
Cholesterol-lowering agents |
1 (3.6) |
|
Alopecia treatment agents |
1 (3.6) |
|
Thyroid disorder medications |
1 (3.6) |
|
Benign prostatic hyperplasia medications |
1 (3.6) |
Table 2.Changes in gut health and bowel movement characteristics before and after the program
|
Category |
Pre (n = 28) |
Post (n = 28) |
x2
|
P-value |
|
Gut health condition |
|
|
|
|
|
No issues |
9 (32.1) |
12 (42.9) |
- |
- |
|
Mild issues |
11 (39.3) |
14 (50.0) |
|
Severe issues |
8 (28.6) |
2 (7.1) |
|
Stool consistency |
|
|
|
|
|
Pellet-like, very hard |
1 (3.6) |
1 (3.6) |
- |
- |
|
Hard pellets but forms a lump |
1 (3.6) |
0 (0.0) |
|
Like a sausage with cracks on the surface |
7 (25.0) |
4 (14.3) |
|
Like a smooth and soft sausage |
9 (32.1) |
11 (39.3) |
|
Soft, lumpy pieces |
7 (25.0) |
9 (32.1) |
|
Mushy, scattered in the toilet bowl |
2 (7.1) |
3 (10.7) |
|
Watery |
1 (3.6) |
0 (0.0) |
|
Defecation frequency |
|
|
|
|
|
Daily |
17 (60.7) |
14 (50.0) |
2.80 |
0.423 |
|
5–6 times/week |
5 (17.9) |
9 (32.1) |
|
3–4 times/week |
6 (21.4) |
5 (17.9) |
|
1–2 times/week |
0 (0.0) |
0 (0.0) |
|
Less than 3 times/month |
0 (0.0) |
0 (0.0) |
|
Time spent on defecation |
|
|
|
|
|
Immediately |
4 (14.3) |
3 (10.7) |
4.533 |
0.475 |
|
Within 5 minutes |
12 (42.9) |
13 (46.4) |
|
5–10 minutes |
6 (21.4) |
9 (32.1) |
|
10–15 minutes |
3 (10.7) |
1 (3.6) |
|
15–30 minutes |
3 (10.7) |
2 (7.1) |
|
More than 30 minutes |
0 (0.0) |
0 (0.0) |
|
Stool volume |
|
|
|
|
|
Less than 1 cup |
4 (14.3) |
1 (3.6) |
- |
- |
|
1–2 cups |
16 (57.1) |
21 (75.0) |
|
2–3 cups |
7 (25.0) |
5 (17.9) |
|
3–4 cups |
0 (0.0) |
1 (3.6) |
|
More than 5 cups |
1 (3.6) |
0 (0.0) |
|
Unknown due to watery stool |
0 (0.0) |
0 (0.0) |
Table 3.Assessment of gut health status changes among participants post-program
|
Category |
Subject (n = 28) |
|
Gut health change |
|
|
Significantly worsened |
0 (0.0) |
|
Worsened |
0 (0.0) |
|
Slightly worsened |
2 (7.1) |
|
No change |
3 (10.7) |
|
Slightly improved |
14 (50.0) |
|
Improved |
6 (21.4) |
|
Significantly improved |
3 (10.7) |
Table 4.Participant satisfaction with overall program
|
Category |
Subject (n = 28) |
|
Overall program satisfaction |
|
|
Very satisfied |
13 (46.4) |
|
Satisfied |
12 (42.9) |
|
Neutral |
3 (10.7) |
|
Dissatisfied |
0 (0.0) |
|
Very dissatisfied |
0 (0.0) |
Table 5.Evaluation of participant perceptions on the effectiveness of interventions for blood glucose and gut health management
|
Questions |
Subject (n = 28) |
|
Do you think that consuming ‘Selex Sunfiber Guar gum Prebiotics (Sunfiber®)’ helped with blood glucose management? |
|
|
Strongly agree |
4 (14.3) |
|
Agree |
15 (53.6) |
|
Neutral |
7 (25.0) |
|
Disagree |
2 (7.1) |
|
Strongly disagree |
0 (0.0) |
|
Do you think that nutritional coaching on dietary intake was helpful for blood glucose management? |
|
|
Strongly agree |
9 (32.1) |
|
Agree |
15 (53.6) |
|
Neutral |
4 (14.3) |
|
Disagree |
0 (0.0) |
|
Strongly disagree |
0 (0.0) |
|
Do you think that the use of continuous glucose monitoring was helpful for blood glucose management? |
|
|
Strongly agree |
15 (53.6) |
|
Agree |
13 (46.4) |
|
Neutral |
0 (0.0) |
|
Disagree |
0 (0.0) |
|
Strongly disagree |
0 (0.0) |
|
Do you think that consuming ‘Selex Sunfiber Guar gum Prebiotics (Sunfiber®)’ contributed to gut health improvement? |
|
|
Strongly agree |
9 (32.1) |
|
Agree |
13 (46.4) |
|
Neutral |
6 (21.4) |
|
Disagree |
0 (0.0) |
|
Strongly disagree |
0 (0.0) |
REFERENCES
- 1. Lee SK, Shin DH, Kim YH, Lee KS. Effect of diabetes education through pattern management on self-care and self-efficacy in patients with type 2 diabetes. Int J Environ Res Public Health 2019; 16(18): 3323.ArticlePubMedPMC
- 2. Korean Diabetes Association (KDA). Diabetes Fact Sheet in Korea 2024. KDA; 2024. p. 1-71.
- 3. National Health Information Potal. Diabetes [Internet]. Korea Disease Control and Prevention Agency; 2020 [updated 2024 Sep 9; cited 2024 Nov 15]. Available from: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do?cntnts_sn=5305
- 4. Kwon HS. Prevalence and treatment status of diabetes mellitus in Korea. J Korean Med Assoc 2023; 66(7): 404-407. ArticlePDF
- 5. Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 2008; 138(3): 439-442. ArticlePubMed
- 6. Post RE, Mainous AG 3rd, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med 2012; 25(1): 16-23. Article
- 7. Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342(19): 1392-1398. ArticlePubMed
- 8. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010; 2(12): 1266-1289. ArticlePubMedPMC
- 9. Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci (Qassim) 2017; 11(2): 65-71.
- 10. Lyu YS, Kim JH, Kim SY. Gut microbiota and diabetes. J Korean Diabetes 2024; 25(3): 117-123. ArticlePDF
- 11. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017; 8(2): 172-184. ArticlePubMedPMCPDF
- 12. Chen M, Yang R, Wang Y, Jia Y, Liu J, Wang G. Non-linear associations of body mass index with impaired fasting glucose, β-cell dysfunction, and insulin resistance in nondiabetic Chinese individuals: a cross-sectional study. Endokrynol Pol 2021; 72(6): 618-624. ArticlePubMed
- 13. Rhee SY, Chon S, Ahn KJ, Woo JT; Korean Diabetes Prevention Study Investigators. Hospital-based Korean Diabetes prevention study: a prospective, multi-center, randomized, open-label controlled study. Diabetes Metab J 2019; 43(1): 49-58. ArticlePubMedPDF
- 14. Menezes MC, Duarte CK, Costa DVP, Lopes MS, Freitas PP, Campos SF, et al. A systematic review of effects, potentialities, and limitations of nutritional interventions aimed at managing obesity in primary and secondary health care. Nutrition 2020; 75-76: 110784.ArticlePubMed
- 15. Saslow LR, Mason AE, Kim S, Goldman V, Ploutz-Snyder R, Bayandorian H, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res 2017; 19(2): e36.ArticlePubMedPMC
- 16. Kim MS, Ryu JM, Kang M, Park J, Ahn YC, Kim YS. Development and adaptability of smartphone-based dietary coaching program for patients undergoing diabetes and prediabetes with continuous glucose monitoring device. J Health Info Stat 2023; 48(1): 36-50. ArticlePDF
- 17. Shreck E, Gonzalez JS, Cohen HW, Walker EA. Risk perception and self-management in urban, diverse adults with type 2 diabetes: the improving diabetes outcomes study. Int J Behav Med 2014; 21(1): 88-98. ArticlePubMedPMCPDF
- 18. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016; 99(6): 926-943. ArticlePubMed
- 19. Yuan C, Lai CW, Chan LW, Chow M, Law HK, Ying M. The effect of diabetes self-management education on body weight, glycemic control, and other metabolic markers in patients with type 2 diabetes mellitus. J Diabetes Res 2014; 2014: 789761.ArticlePubMedPMCPDF
- 20. Kim Y, Park JE, Lee BW, Jung CH, Park DA. Comparative effectiveness of telemonitoring versus usual care for type 2 diabetes: a systematic review and meta-analysis. J Telemed Telecare 2019; 25(10): 587-601. ArticlePubMedPDF
- 21. Jiang Y, Ramachandran HJ, Teo JYC, Leong FL, Lim ST, Nguyen HD, et al. Effectiveness of a nurse-led smartphone-based self-management programme for people with poorly controlled type 2 diabetes: a randomized controlled trial. J Adv Nurs 2022; 78(4): 1154-1165. ArticlePubMed
- 22. Korean Diabetes Association (KDA). 2023 Clinical Practice Guidelines for Diabetes [Internet]. KDA; 2023 [cited 2025 Jan 8]. Available from: https://diabetes.or.kr/bbs/?code=guide&mode=view&number=1284&page=1&code=guide
- 23. Ministry of Food and Drug Safety. Ingredient-specific information for health functional foods [Internet]. Food Safety Korea; 2015 [cited 2024 Nov 14]. Available from: http://www.foodsafetykorea.go.kr/
- 24. Yook SM, Lim YS, Lee JS, Kim KN, Hwang HJ, Kwon S, et al. Revision of nutrition quotient for Korean adults: NQ-2021. J Nutr Health 2022; 55(2): 278-295. ArticlePDF
- 25. Choi YJ, Cho JH, Lee DH, Song DJ, Kwon YJ, Baek SM, et al. Development of Koreans gut quotient measurement scales. Korean J Gastroenterol 2019; 73(6): 341-349. ArticlePubMedPDF
- 26. Wolever TM. Relationship between dietary fiber content and composition in foods and the glycemic index. Am J Clin Nutr 1990; 51(1): 72-75. ArticlePubMed
- 27. Nishimune T, Yakushiji T, Sumimoto T, Taguchi S, Konishi Y, Nakahara S, et al. Glycemic response and fiber content of some foods. Am J Clin Nutr 1991; 54(2): 414-419. ArticlePubMed
- 28. Lee C, Shin JS. Effects of different fiber content of rice on blood glucose and triglyceride levels in normal subject. J Korean Soc Food Sci Nutr 2002; 31(6): 1048-1051. Article
- 29. Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, et al. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr 2005; 82(6): 1185-1194. ArticlePubMed
- 30. Oh K, Hu FB, Cho E, Rexrode KM, Stampfer MJ, Manson JE, et al. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. Am J Epidemiol 2005; 161(2): 161-169. ArticlePubMed
- 31. Lee YS, Lee SY. The association between dietary fiber intake and prevalence of metabolic syndrome in middle-aged adults in Gyeonggi province. Korean J Health Promot 2015; 15(2): 75-82. Article
- 32. National Health Information Potal. Dietary nutrition [Internet]. Korea Disease Control and Prevention Agency; 2020 [updated 2024 Dec 26; cited 2024 Dec 30]. Available from: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do?cntnts_sn=5298
- 33. Yu KH, Chung CE, Cho SS, Ly SY. Analysis of dietary fiber intake in the Korean adult population using 2001 Korean National Health and Nutrition Survey data and newly established dietary fiber database. Korean J Nutr 2008; 41(1): 100-110.
- 34. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care 2011; 34(6): 1323-1328. ArticlePubMedPMC
- 35. Vuorinen-Markkola H, Sinisalo M, Koivisto VA. Guar gum in insulin-dependent diabetes: effects on glycemic control and serum lipoproteins. Am J Clin Nutr 1992; 56(6): 1056-1060. ArticlePubMed
- 36. Landin K, Holm G, Tengborn L, Smith U. Guar gum improves insulin sensitivity, blood lipids, blood pressure, and fibrinolysis in healthy men. Am J Clin Nutr 1992; 56(6): 1061-1065. ArticlePubMed
- 37. Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. J Diabetes Investig 2018; 9(4): 713-725. ArticlePubMedPMCPDF
- 38. Nelson LA, Wallston KA, Kripalani S, Greevy RA Jr, Elasy TA, Bergner EM, et al. Mobile phone support for diabetes self-care among diverse adults: protocol for a three-arm randomized controlled trial. JMIR Res Protoc 2018; 7(4): e92. ArticlePubMedPMC
Citations
Citations to this article as recorded by

- Comparative analysis of dietary and lifestyle habits according to the prediabetic status in young adults
Joungyoon Seo, SeongHee Shin, Yuri Kim, Yoo Kyoung Park
Journal of Nutrition and Health.2025; 58(5): 468. CrossRef - Partially Hydrolyzed Guar Gum Combined with a Low-Fat Diet Ameliorates Type 2 Diabetes Mellitus via Modulating Gut Microbiota and Fecal Metabolites
Zhiqiang Cao, Hongxia Li, Quantao Cai, Li Chen, Liangzhong Liu, Yuhan Tang, Zhe Zhu, Ping Yao
Nutrients.2025; 17(23): 3746. CrossRef
 , A-Hyun Jeong2)
, A-Hyun Jeong2) , Hyejung Hong3)
, Hyejung Hong3) , Hana Jang4)
, Hana Jang4) , Hye-Jin Kim5),†
, Hye-Jin Kim5),†






 KSCN
KSCN

 Cite
Cite