Articles
- Page Path
- HOME > Korean J Community Nutr > Volume 19(1); 2014 > Article
-
Original Article
- Association of Food and Nutrient Intakes with Periodontitis by Smoking Status among Korean Adults
- Sunghee Kim, Areum Yu, Yoon Jung Yang
-
Korean Journal of Community Nutrition 2014;19(1):84-94.
DOI: https://doi.org/10.5720/kjcn.2014.19.1.84
Published online: February 28, 2014
Department of Clinical Nutrition, Dongduk Women's University, Seoul, Korea.
1Department of Food and Nutrition, Dongduk Women's University, Seoul, Korea.
- Corresponding author: Yoon Jung Yang, Department of Food and Nutrition, Dongduk Women's University, 13 Hwarang-ro, Seongbukgu, Seoul 136-714, Korea. Tel: (02) 940-4465, Fax: (02) 940-4193, yjyang@dongduk.ac.kr
Copyright © 2014 The Korean Society of Community Nutrition
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,288 Views
- 4 Download
- 1 Crossref
Figure & Data
REFERENCES
Citations

- Association between consumption of milk and dairy products, calcium and riboflavin, and periodontitis in Korean adults: Using the 2007-2010 Korea National Health and Nutrition Examination Surveys
Sang Mi Koo, Deog-Gyu Seo, Yoon Jung Park, Ji-Yun Hwang
Journal of Nutrition and Health.2014; 47(4): 258. CrossRef

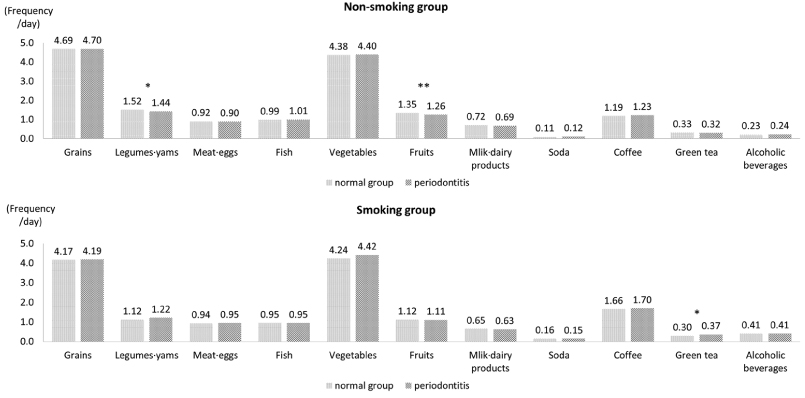
Fig. 1
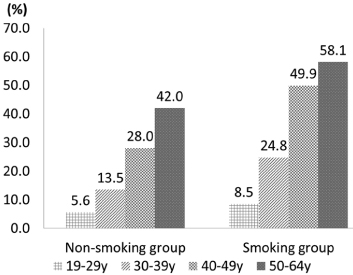
Fig. 2
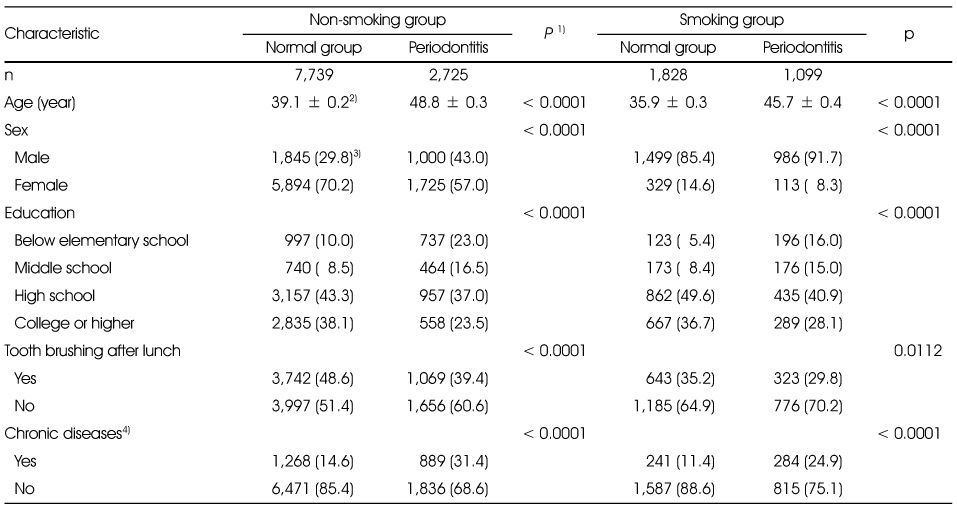
General characteristics of the study subjects and by the periodontitis by smoking status
1) t-test for continuous variables and χ2-test for categorical variables
2) Mean ± SD
3) N (%)
4) Chronic diseases were defined as including one of more of diabetes, dyslipidemia, hypertension, angina or myocardial infarction
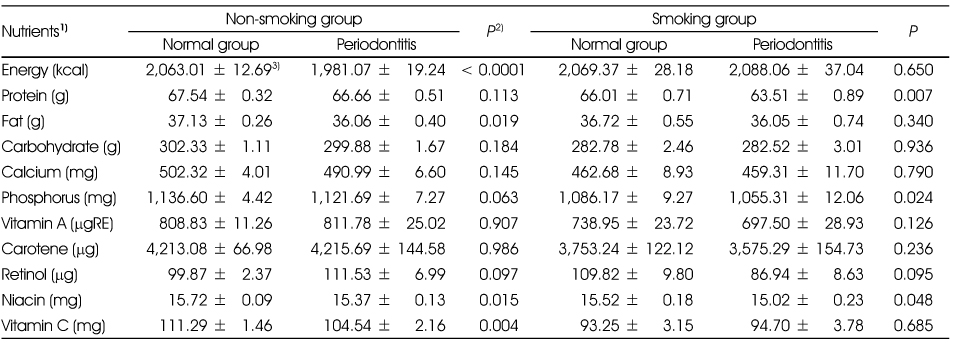
Nutrient intakes of the subjects according to periodontitis by the smoking status
1) All nutrients except energy were total energy adjusted by residual method after log transformation.
2) t-test
3) Mean ± SD
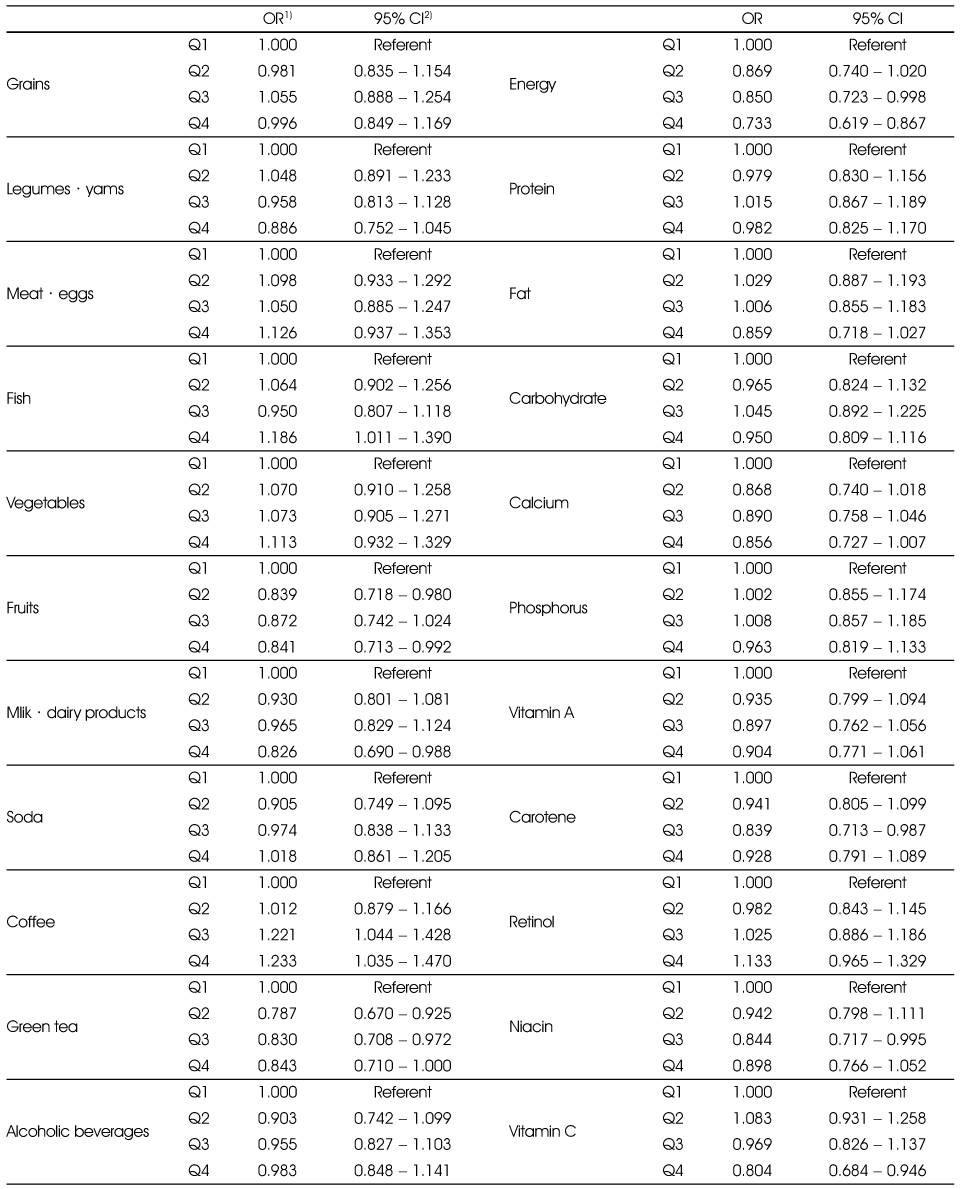
Adjusted ORs and 95% CIs between the prevalence of periodontitis and food and nutrient intakes in the non-smoking group
Multiple logistic regression analysis after adjusting for age, sex education (elementary, middle, high, college), tooth brushing after lunch (yes, no), and chronic diseases (yes, no).
1) Odds Ratio
2) 95% confidence interval
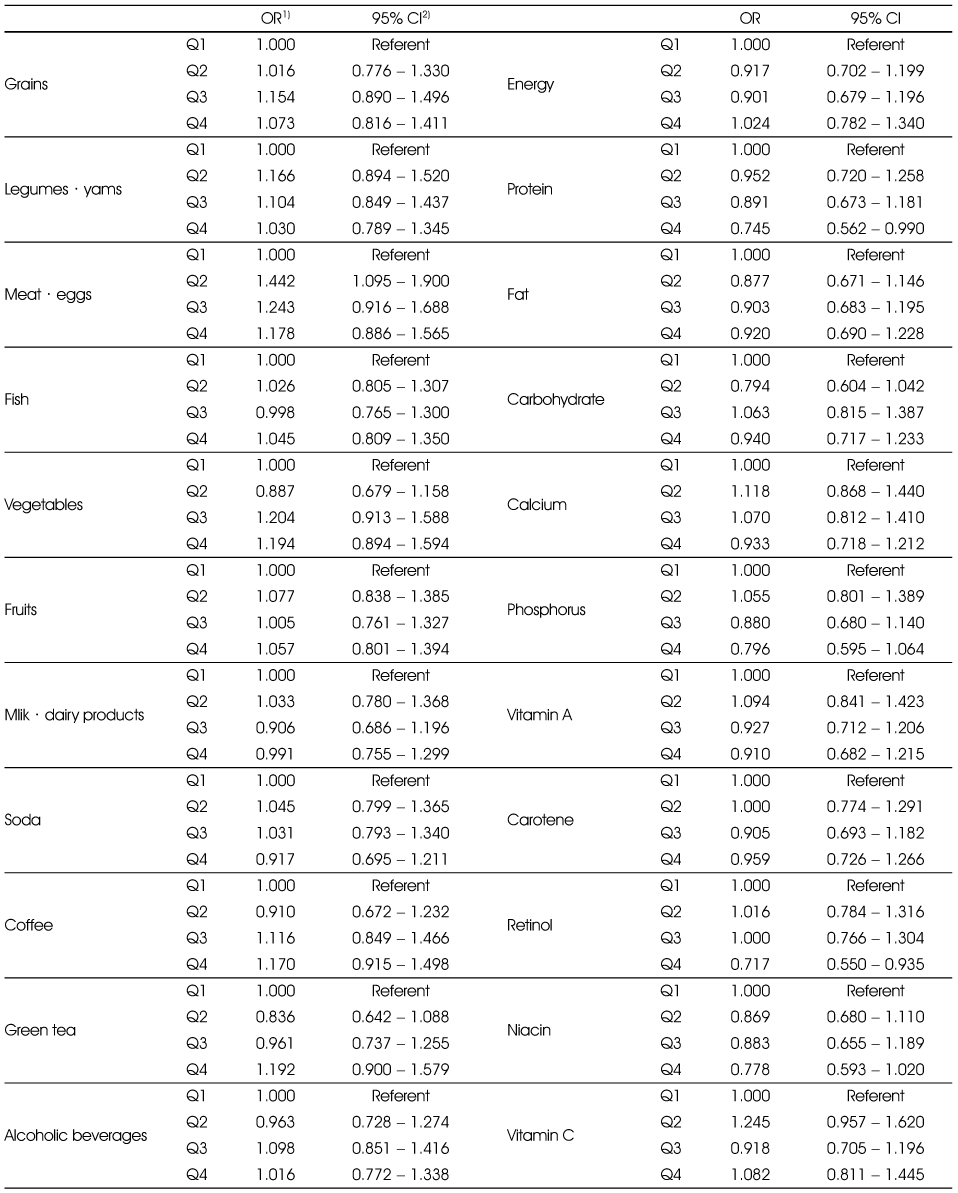
Adjusted ORs and 95% CIs between the prevalence of periodontitis and food and nutrient intakes in the smoking group
Multiple logistic regression analysis after adjusting for age, sex education (elementary, middle, high, college), tooth brushing after lunch (yes, no), and chronic disease (yes, no).
1) Odds Ratio
2) 95% confidence interval
1) t-test for continuous variables and χ2-test for categorical variables 2) Mean ± SD 3) N (%) 4) Chronic diseases were defined as including one of more of diabetes, dyslipidemia, hypertension, angina or myocardial infarction
1) All nutrients except energy were total energy adjusted by residual method after log transformation. 2) t-test 3) Mean ± SD
Multiple logistic regression analysis after adjusting for age, sex education (elementary, middle, high, college), tooth brushing after lunch (yes, no), and chronic diseases (yes, no). 1) Odds Ratio 2) 95% confidence interval
Multiple logistic regression analysis after adjusting for age, sex education (elementary, middle, high, college), tooth brushing after lunch (yes, no), and chronic disease (yes, no). 1) Odds Ratio 2) 95% confidence interval

 KSCN
KSCN

 Cite
Cite